Preparation method of supported nickel-based catalyst for synthesizing chloroaniline through selective hydrogenation of chloronitrobenzene
A chloronitrobenzene, nickel-based catalyst technology, applied in the preparation of amino compounds, the preparation of organic compounds, metal/metal oxide/metal hydroxide catalysts and other directions, can solve the problem of expensive, difficult to recover, easy to deactivate and other problems, to achieve the effects of low production cost, low dechlorination performance and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation method of the supported nickel catalyst for the selective catalytic hydrogenation of chloronitrobenzene comprises the following steps:
[0033] (1) First, γ-Al 2 o 3 Roast at 500°C for 5h;
[0034] (2) Weigh 2g of γ-Al after roasting 2 o 3 and 0.9908g nickel nitrate (Ni(NO 3 ) 2 ·6H 2 O) Add it to 50mL of ethylene glycol, raise the temperature to 90°C under stirring, keep it for 30min, then add 0.2mol / L Na2 CO 3 The aqueous solution was brought to pH=9-10 to obtain a suspension.
[0035] The suspension was aged at 25°C for 2 hours, filtered and washed until the filtrate was neutral, then dried at 120°C for 12 hours, and ground through a 100-mesh sieve to obtain a solid powder;
[0036] (3) Calcining the obtained solid powder in air at 400° C. for 4 hours to obtain a catalyst precursor.
[0037] (4) Place the obtained catalyst precursor in a tubular heating furnace and reduce it under a high-purity hydrogen atmosphere with a flow rate of 20mL / min...
Embodiment 2
[0041] Steps (1)-(2) are the same as in Example 1.
[0042] (3) Calcining the obtained solid powder at 500° C. for 4 hours to obtain a catalyst precursor.
[0043] (4) Place the obtained catalyst precursor in a tubular heating furnace and reduce it under a high-purity hydrogen atmosphere with a flow rate of 20mL / min. The reduction temperature is 300°C and the reduction time is 1h. A supported nickel catalyst for the selective hydrogenation of benzene, designated Cat-2.
[0044] The evaluation of the above-mentioned catalysts is the application of the catalysts:
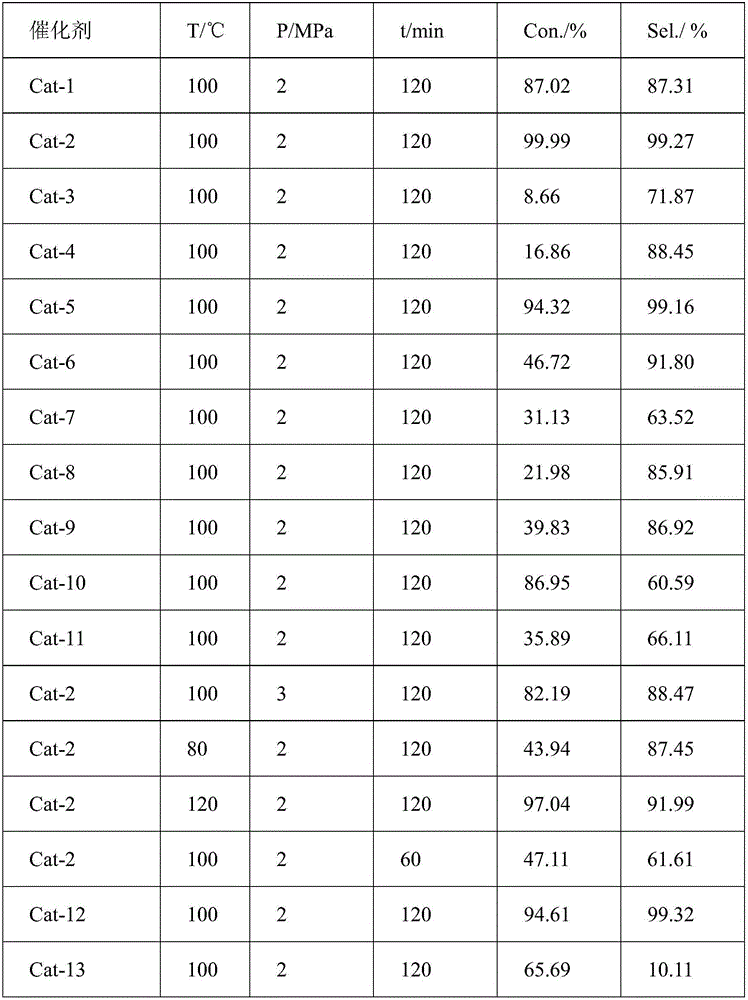
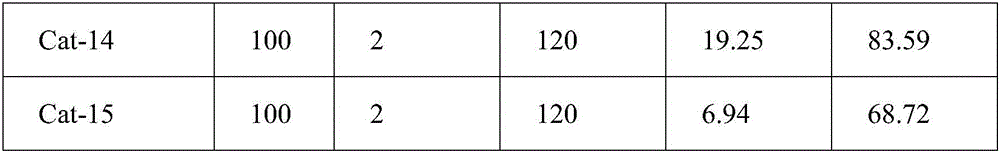
[0045] Under the conditions of 100°C, hydrogen pressure 2.0Mpa, 1.0000g o-chloronitrobenzene, 0.4000g Cat-2 catalyst, and 20mL absolute ethanol, react for 120min to obtain o-chloroaniline. The reaction results are shown in Table 1.
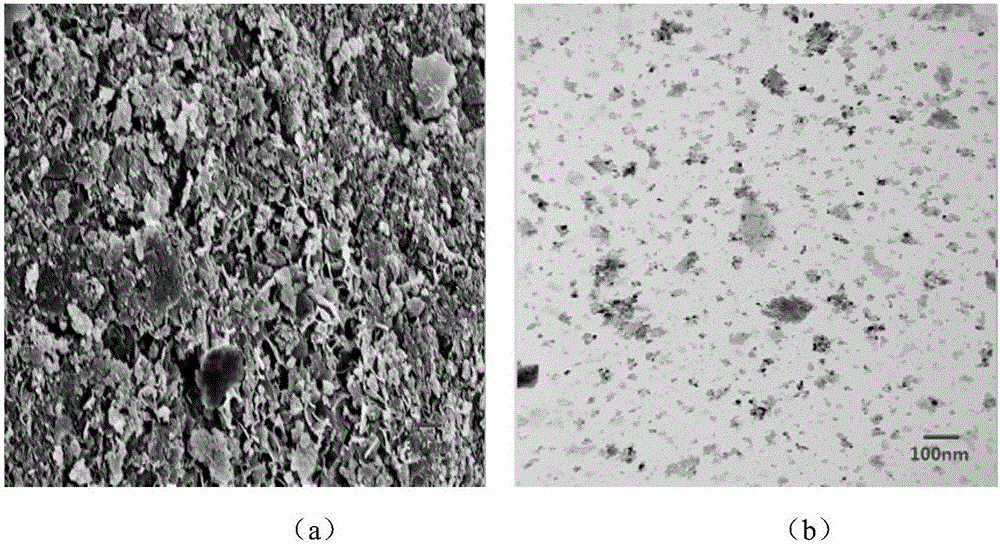
[0046] figure 1 (a) is NiO / γ-Al after calcination at 500°C for 4 hours (i.e. Example 2) 2 o 3 SEM spectra of catalyst precursors. Observing the SEM image of the sample, it can be s...
Embodiment 3
[0049] Steps (1)-(2) are the same as in Example 1.
[0050] (3) Calcining the obtained solid powder at 600° C. for 4 hours to obtain a catalyst precursor.
[0051] (4) Place the obtained catalyst precursor in a tubular heating furnace and reduce it under a high-purity hydrogen atmosphere with a flow rate of 20mL / min. The reduction temperature is 300°C and the reduction time is 1h. A supported nickel catalyst for the selective hydrogenation of benzene, designated Cat-3.
[0052] The evaluation of the above-mentioned catalysts is the application of the catalysts:
[0053] Under the conditions of 100°C, hydrogen pressure 2.0Mpa, 1.0000g o-chloronitrobenzene, 0.4000g Cat-3 catalyst, and 20mL absolute ethanol, react for 120min to obtain o-chloroaniline. The reaction results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com