Preparation method of meloxicam transdermal patch
A technology of transdermal patch and meloxicam, applied in the field of pharmacy, can solve the problem of difficulty in realizing long-term uniform release of meloxicam, and achieve the effects of eliminating defects, avoiding toxic and side effects, and facilitating administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
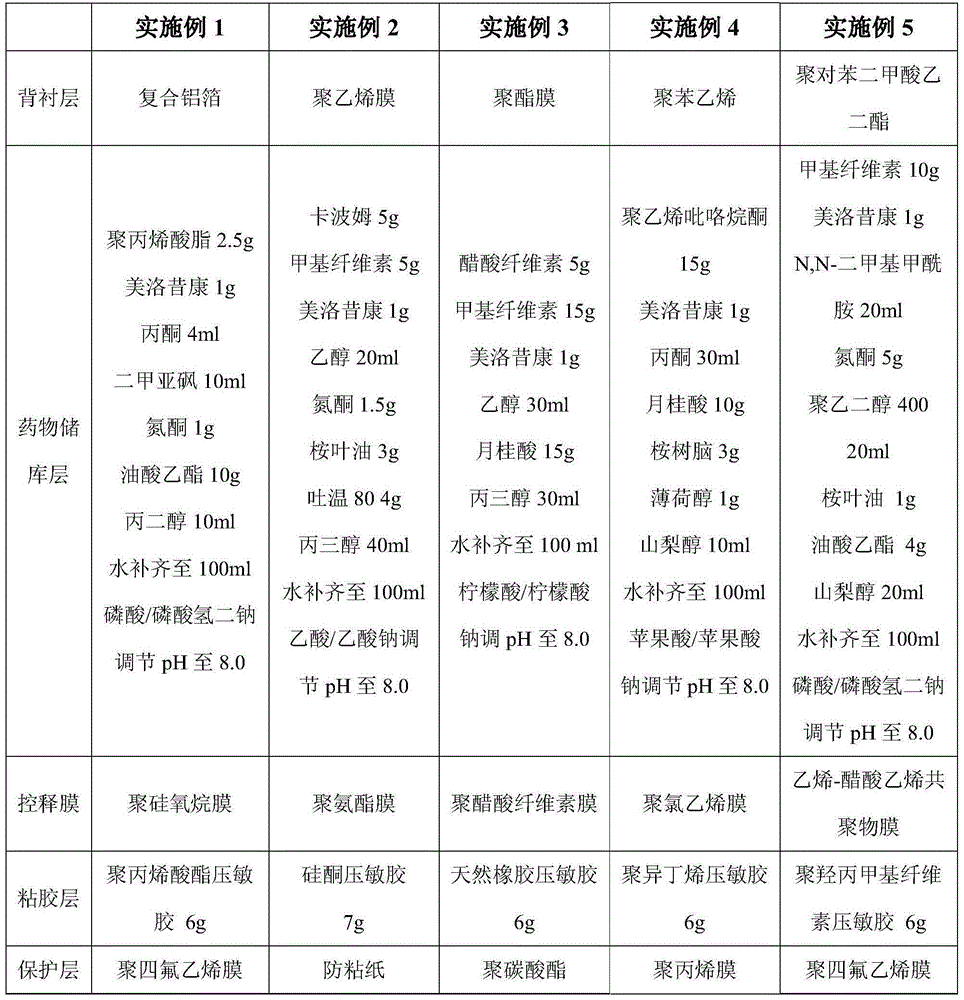
Examples
preparation example Construction
[0035] 2. Preparation of the adhesive layer: fully dissolve the pressure-sensitive adhesive specified in the prescription in an organic solvent, and evenly coat it on the controlled release film, heat and dry the organic solvent at 40°C, and cover it with a protective layer for later use.
[0036] 3. Preparation of transdermal patch: evenly add the drug reservoir to the controlled release film, dry for 12 hours at 25°C, cover with the backing layer, heat seal, and cut it. The backing layer, controlled release film and protective layer are all 20×50cm 2 , The transdermal meloxicam patch of the present invention is all cut to 5×5cm 2 Store for later use, that is, the content of meloxicam is 1mg / cm 2 , In actual use, the specifications can be tailored according to the size, weight and severity of the disease.
Embodiment 6
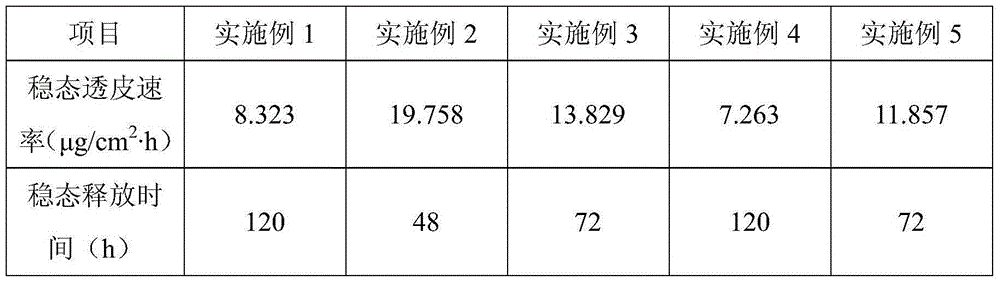
[0037] Example 6: In vitro transdermal experiment
[0038] Release measurement: the nude mouse was sacrificed by severed neck, the skin was removed, and it was laid flat on a clean glass plate with the cuticle facing down to remove the subcutaneous fat tissue and adhesions. After repeated washing with normal saline, it was placed in normal saline for use. . After peeling off the protective layer of the meloxicam patch (Examples 1-5), stick it on the side with the stratum corneum, and then fix the mouse skin between the two halves of the diffusion tank, with the stratum corneum facing the supply tank. Add 5ml of phosphate buffer with pH 8 as the receiving medium to each receiving pool, sample 1ml regularly, and add 1ml blank medium at the same time. The concentration of meloxicam in the sample was determined by high performance liquid chromatography (HPLC method).
[0039] The meloxicam patch prepared by the above method can reach a stable release within 30 minutes. The specific e...
Embodiment 7
[0043] Example 7: In vivo pharmacokinetic test
[0044] Thirty-six Wistar male rats, weighing about 200±20g, were randomly divided into 2 groups. The first group was given 6 rats by intragastric administration of 2 mg meloxicam, and the second group was 30 rats. The meloxicam patch equivalent to 2 mg was administered to the joints (Example 1-5; 2cm 2 ×1mg / cm 2 ), fasting 12 hours before and after administration. At a specified time point after administration, 3 mL of blood was taken from the orbit of the rat, the plasma was separated, and the concentration of meloxicam in the sample was determined by high performance liquid chromatography (HPLC method). The in vivo pharmacokinetic test data is shown in Table 3 below:
[0045] Table 3 Example 1-5 Meloxicam transdermal patch in vivo kinetics test results
[0046]
[0047]
[0048] After oral administration, the drug reaches its peak after 4 hours in the blood, C max =22.35±9.77μg / ml, and then decreased with time. After topical adm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com