Photosensitive dopant, preparation method thereof, self-assembly system and photoresponse device
A dopant and self-assembly technology, applied in the field of light regulation, can solve the problems of inapplicability, high synthesis cost, and small molecular size of photosensitizers, and achieve the effects of high ultraviolet absorption capacity, high molecular deformation index, and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
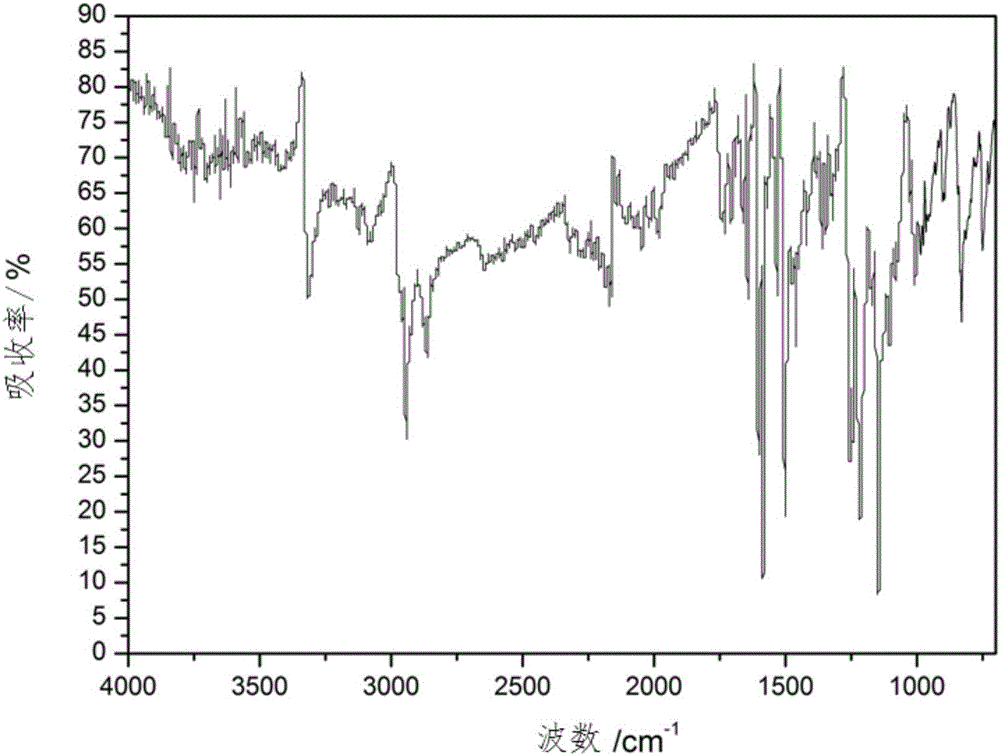
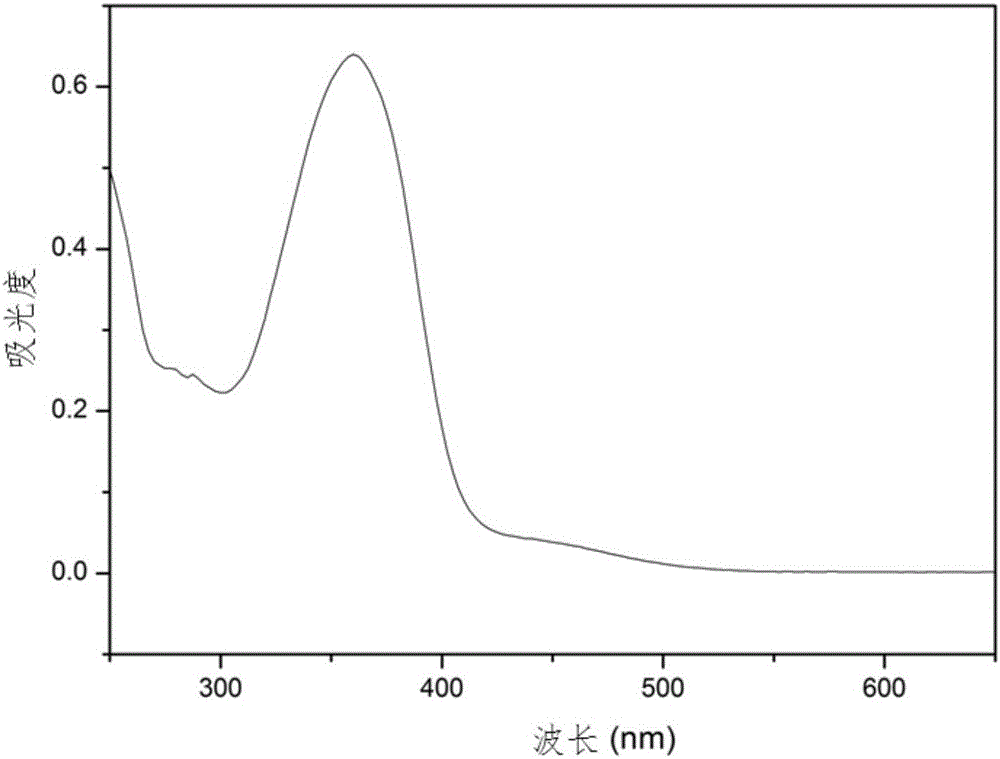
Image
Examples
Embodiment 1
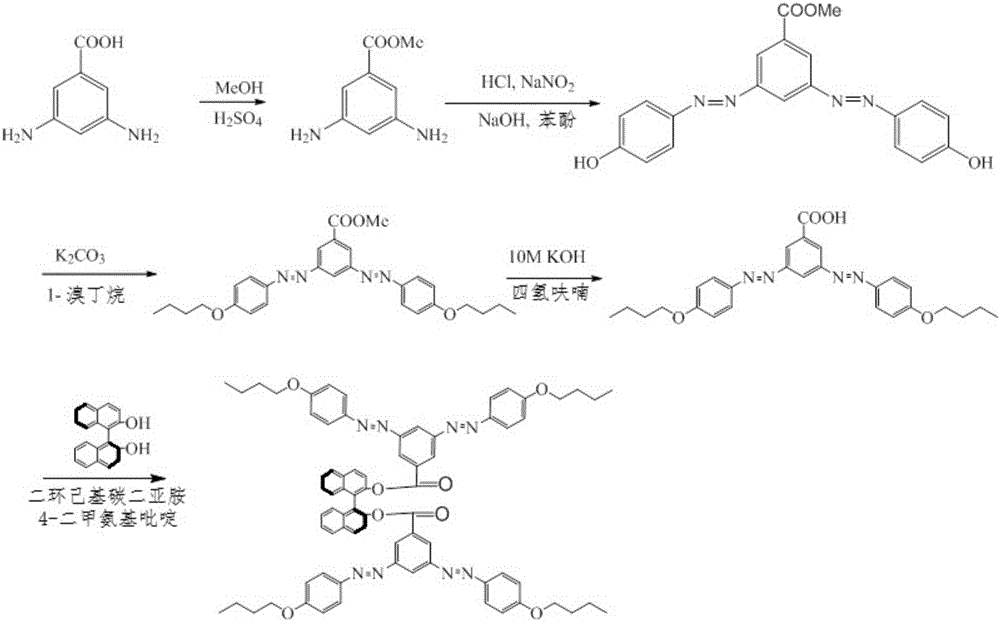
[0036] refer to figure 1 The synthetic route map of carrying out the synthesis of photoactive dopant:
[0037] (1) Add 10g of 3,5-di-aminobenzoic acid and 100mL of methanol into a single-necked flask, start stirring, slowly drop 5mL of concentrated sulfuric acid, and then reflux for 20-30 hours. After stopping the reaction, most of the solvent was evaporated by rotary evaporation, and then an appropriate amount of ethyl acetate and saturated sodium bicarbonate solution was added to make the pH of the system between 7-9, and the organic phase was obtained by liquid separation, and dried over sodium sulfate. Rotary evaporation gave methyl 3,5-di-aminobenzoate.
[0038] (2) Place 1 g of methyl 3,5-di-aminobenzoate in a beaker under ice-bath conditions, add 30 mL of water and 3.7 mL of concentrated hydrochloric acid; and prepare 5 mL of 0.824 g of sodium nitrite aqueous solution. Slowly drop the solution containing sodium nitrite into the beaker and react for 30 minutes.
[003...
Embodiment 2
[0044] refer to Figure 5 The synthetic route map of carrying out the synthesis of photoactive dopant:
[0045] Carry out the steps (1)-(5) identical with embodiment 1;
[0046] (6) Weigh 2g of R-binaphthol, 1.5g of potassium carbonate and 0.1g of potassium iodide in a single-necked flask, add 30mL of acetone to dissolve, start stirring and slowly add 0.751mL of n-bromobutane solution, and reflux for 2 days. Rotary evaporate the organic phase, add 100mL ethyl acetate and 3×100mL aqueous liquid extraction treatment, separate the organic phase, treat with anhydrous sodium sulfate, rotary evaporation, and column chromatography to obtain 2-n-butoxy (R) binaphthol. H NMR (400MHz d6-DMSO):δ=0.690(t,3H),δ=1.01(d,2H),δ=1.36(d,2H),δ=3.99(d,2H),δ=6.88(s ,1H),δ=7.02(s,1H),δ=7.14(s,1H),δ=7.22(d,2H), δ=7.33(d,2H),δ=7.55(s,1H),δ =7.84(d,2H),δ=7.91(s,1H),δ=8.00(s,1H),δ=9.25(s,1H).
[0047] (7) Weigh 0.69g of 3,5-bis-(4-butyl ether phenylazo)benzoic acid and 0.5g of 2-n-butoxy (R) binapht...
Embodiment 3
[0049] refer to Image 6 The synthetic route map of carrying out the synthesis of chiral photosensitive dopant:
[0050] Carry out the steps (1)-(5) identical with embodiment 1;
[0051] (6) Weigh 1g of R-binaphthol, 0.73g of potassium carbonate and 0.045g of potassium iodide into a single-necked flask, add 30mL of acetone to dissolve, start stirring and slowly add 0.45mL of n-bromohexane solution, and reflux for 2 days. Rotary evaporate the organic phase, add 100mL ethyl acetate and 3×100mL aqueous liquid extraction treatment, separate the organic phase, treat with anhydrous sodium sulfate, rotary evaporation, and column chromatography to obtain 2-n-butoxy (R) binaphthol. H NMR (400MHz d6-DMSO):δ=0.70(t,3H),δ=0.85-1.02(m,6H),δ=1.33(d,2H),δ=3.99(d,2H),δ=6.86 (s,1H),δ=7.02(s,1H),δ=7.12-7.33(m,5H),δ=7.55(s,1H),δ=7.91-8.01(m,4H),δ=9.25( s, 1H).
[0052] (7) Weigh 0.50g of 3,5-bis-(4-butyl ether phenylazo) benzoic acid and 0.325g of 2-n-hexyloxy (R) binaphthol, dissolve 25mL o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com