PH response insulin slow release nanoparticle, and preparation method and application thereof
A technology of insulin and nanoparticles, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients. The effect of reducing the amount of drug release and reducing the number of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0045] Synthesis of embodiment 1 triblock polymer mPEG-PCL-PDEAEMA
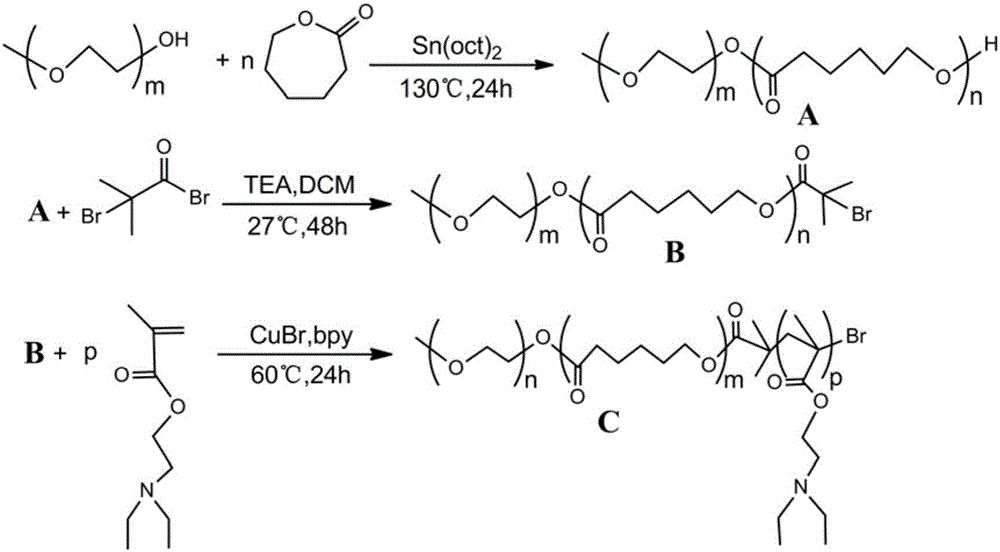
[0046] 1. Synthesis of mPEG-PCL-OH
[0047] By ring-opening polymerization. Accurately weigh an appropriate amount of mPEG5000 and ε-CL (w:w, 1:2-2.6), place it in a 50ml dry round bottom flask, and add 1% ε-CL molar mass of Sn(oct) 2 As a catalyst, react in a nitrogen environment at 130°C for 24 hours. After the reaction, add an appropriate amount of DCM to dilute, precipitate in excess glacial ether, filter with suction, collect the precipitate, and add an appropriate amount of DCM to re-dissolve. The above operation was repeated twice to obtain a white powdery product, which was vacuum-dried at 40° C. to constant weight.
[0048] 2. Synthesis of macromolecular initiator mPEG-PCL-Br
[0049] Accurately weigh an appropriate amount of the above product mPEG-PCL-OH in a 50ml dry round bottom flask, add an appropriate amount of DCM to dissolve, and add TEA and BiBB in proportion to the ice-water bath (w:w:w,...
Embodiment 2
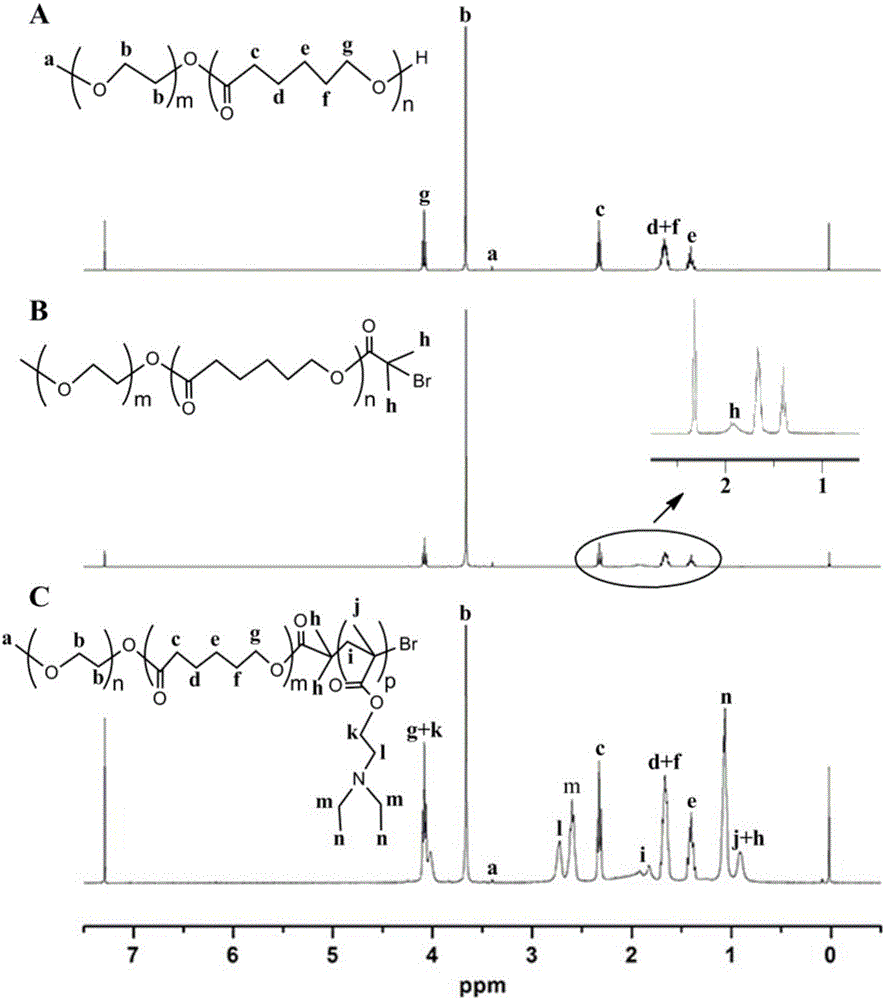
[0052] The characterization of embodiment 2 polymer
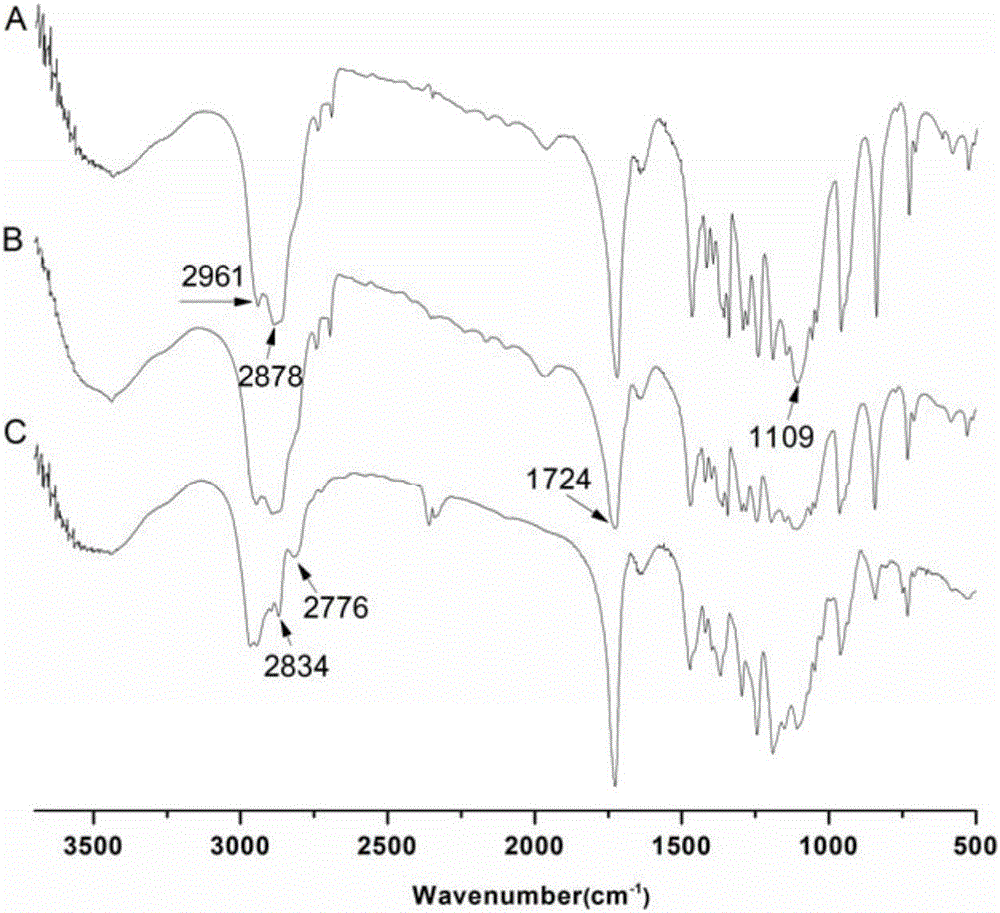
[0053] 1 Fourier Transform Infrared Spectroscopy (FT-IR) Characterization
[0054] Grind an appropriate amount of the product to be tested and dried potassium bromide into powder at room temperature, take an appropriate amount of powder for tableting, and scan 400-4 000 cm -1 Wavenumber Absorption Spectrum.
[0055] Analysis of FT-IR spectrum results
[0056] figure 2 All spectra in 1109cm -1 There is a stretching vibration peak of the C-O bond of the mPEG segment at 1724cm -1 There is a stretching vibration peak of the C=O bond of the PCL chain segment at 2878cm -1 There are mPEG segments and PCL segments at -CH 2 The stretching vibration peak of - indicates the successful synthesis of mPEG and PCL. Due to the small proportion of -C-Br- in mPEG5k-PCL10k-Br, the figure 2 -A and figure 2 -B difference is not obvious. figure 2 -C at 2776cm -1 and 2834cm -1 The unique methyl and methylene stretching peaks of PD...
Embodiment 3
[0067] Example 3 Preparation of polymer-loaded INS nanoparticles
[0068] Polymer nanoparticles were prepared by nanoprecipitation technique. Accurately weigh an appropriate amount of INS and dissolve it in 6ml of 0.01mol / L HCl solution, and adjust its pH value to 6.0 with 1mol / L NaOH solution under the detection of a pH meter. Fully dissolve 6 mg of the polymer in 0.6 ml of tetrahydrofuran, slowly add it dropwise into the above solution under stirring, and stir overnight in a fume hood at room temperature to evaporate the tetrahydrofuran. Centrifuge at 14,000 rpm for 30 min at 4°C, collect the precipitate, wash with deionized water three times, and freeze-dry to obtain polymer-loaded INS nanoparticles. Polymer blank nanoparticles were prepared in the same way.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com