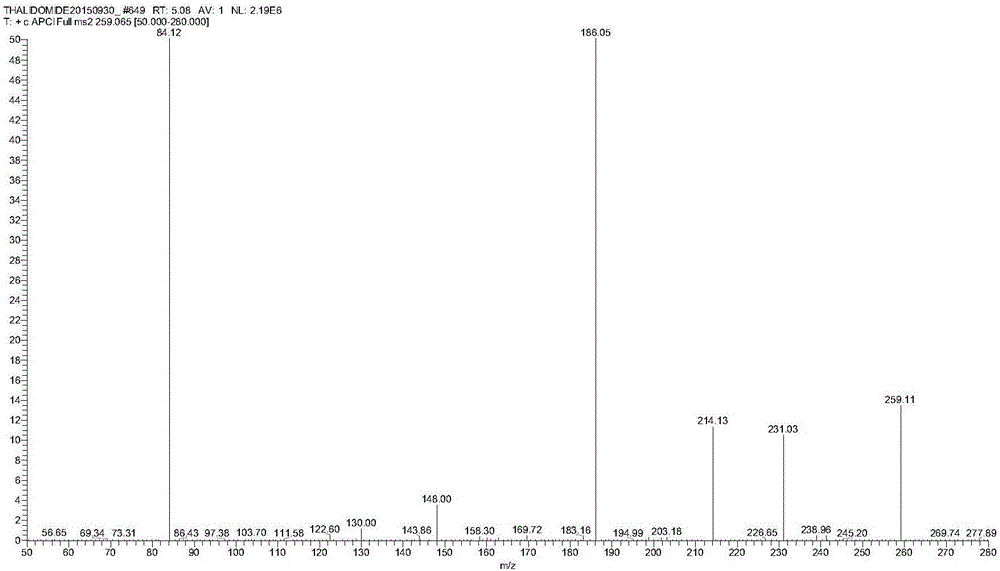
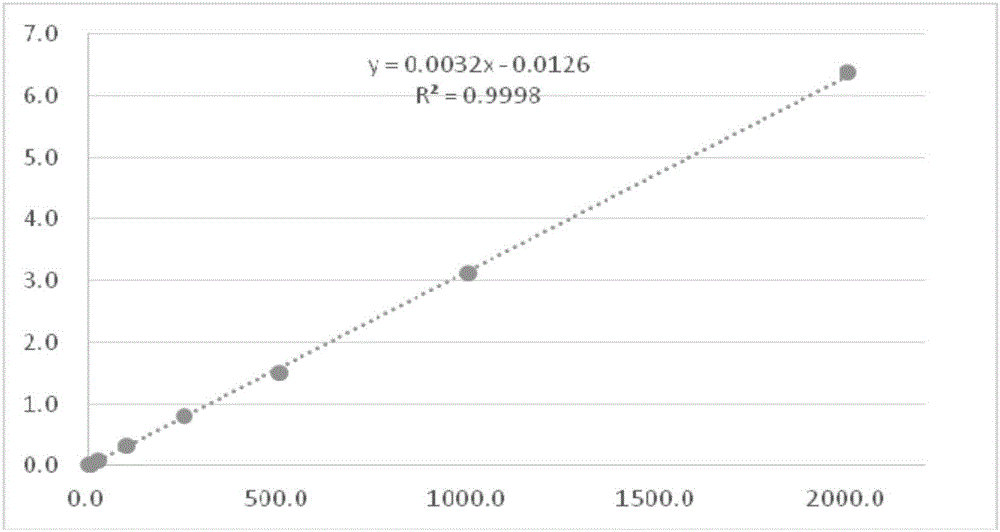
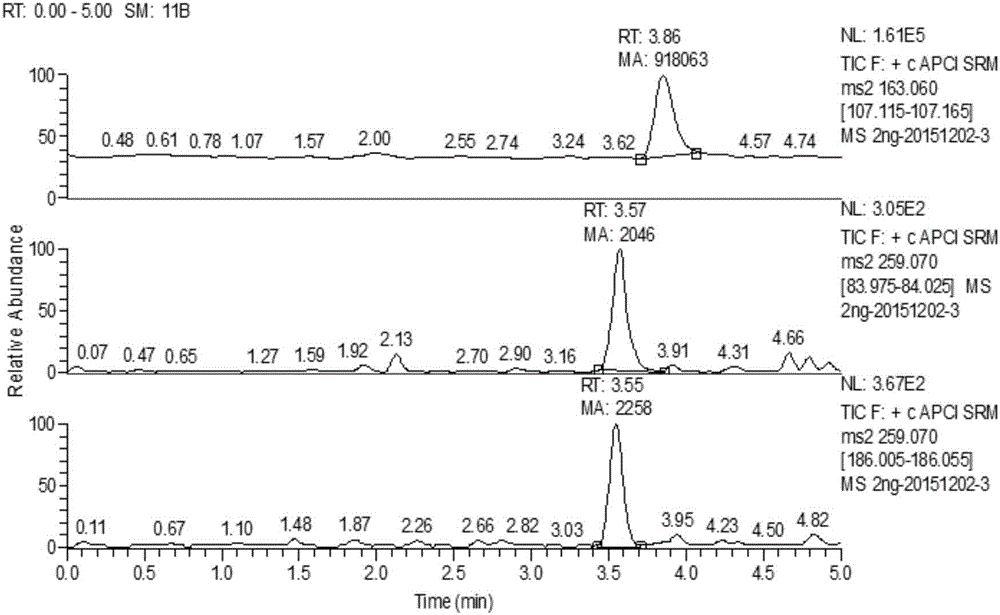
Method for detecting thalidomide in plasma by LC-MS/MS method
A technology of thalidomide and plasma, which is applied in the field of monitoring the drug concentration of the drug and detecting thalidomide in plasma, can solve the problem that the clinical efficacy of thalidomide has no obvious dose relationship, cannot fully meet the needs of clinical determination, and affects Thalidomide precision drug use and other issues, to achieve the effect of short analysis time, short processing time, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Establishment of a standard curve for determining the concentration of thalidomide in plasma
[0047] 1. Preparation of standard working solution:
[0048](1) Accurately weigh 0.0100g of thalidomide standard substance, dissolve it with 1ml DMSO, transfer it into a 10ml volumetric flask, and dilute to the mark with DMSO, shake well to obtain a standard stock solution of 1 000 μg / ml, place in Store in refrigerator at -20°C. Then use methanol: acetonitrile: formic acid (50:49:1, v / v / v) solution gradient dilution to prepare standard working solutions with concentrations of 20, 50, 250, 1000, 2500, 5000, 10000, 20000 ng / ml, Store in refrigerator at 4°C.
[0049] (2) Accurately weigh 0.0050 g of umbelliferin standard substance, dissolve it in methanol, transfer it to a 50 ml volumetric flask, and measure it with methanol: acetonitrile: formic acid (50:49:1, v / v / v). Bring the volume up to the mark, shake well to obtain a 100 μg / ml standard stock solution, and stor...
Embodiment 2
[0064] Example 2 Detection of spiked samples
[0065] 1. Pretreatment of spiked plasma samples
[0066] (1) Venous blood was collected from healthy volunteers. The blood samples were placed in heparinized EP tubes, and the whole blood was centrifuged at 4000 rpm for 5 min to absorb the upper plasma, that is, the blank human plasma, which was divided into 100 µL and stored at -80°C.
[0067] Thalidomide was added to blank human plasma to obtain spiked plasma samples with thalidomide concentrations of 2, 5, 100, and 1600 ng / mL.
[0068] (2) Take 100 µL of the spiked plasma sample, and after processing according to the sample pretreatment in Example 1, take 80 µL of the supernatant and transfer it into the sample bottle.
[0069] 2. Detection of spiked plasma samples
[0070] 10 µL was injected for UPLC-MS / MS analysis, and the conditions were the same as in Example 1. According to the standard curve, the plasma sample concentration is calculated as 2.1±0.3, 5.2±0.5, 107.8±3.6...
Embodiment 3
[0072] Example 3 Sample detection
[0073] 1. Thalidomide concentration in patients with inflammatory bowel disease after taking different doses of thalidomide tablets for 12 hours
[0074] (1) About 2 mL of venous blood was collected from patients with inflammatory bowel disease after taking different doses of thalidomide tablets for 12 hours. Blood samples were placed in heparinized EP tubes, and the whole blood was centrifuged at 4000 rpm for 5 min to absorb the upper layer of plasma, which was divided into 100 µL and stored at -80°C until assayed.
[0075] (2) 100 µL of human plasma sample was pretreated with the sample pretreatment method described in Example 1, and 80 µL of the supernatant was taken and transferred into the sample bottle.
[0076] 2. Concentration study of plasma samples in patients with inflammatory bowel disease after taking different doses of thalidomide tablets for 12 hours
[0077] 10 µL was injected for UPLC-MS / MS analysis, the conditions were th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com