Long-wavelength fluorescent probe for detecting hydrazine and synthetic method and application of long-wavelength fluorescent probe
A synthesis method and fluorescent molecular probe technology, applied in the field of chemical analysis and detection, can solve the problems of weak photobiological penetration ability and unfavorable biological sample detection, etc., and achieve good anti-interference ability, stable optical performance and fast response speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of Fluorescent Molecular Probes
[0048] Compound 3 (R 1 , R 2 Both are alkyl groups with one carbon number) (398 mg, 2.0 mmol) and p-Hydroxybenzaldehyde (268 mg, (2.2 mmol) were added to ethanol (50 mL) and heated under reflux for 12 h. After the reaction, distillation under reduced pressure The solvent was removed and separated by column chromatography to obtain a brown-red solid (compound 2).
[0049] Compound 2 (303 mg, 1 mmol), acetyl chloride (157 mg, 2.0 mmol, 142 mL) was added to triethylamine (304 mg, 2.2 mmol, 416 mL), and the solvent methylene chloride (20 mL) was reacted at room temperature for 8 h . After the reaction was completed, the solvent was distilled off under reduced pressure and separated by column chromatography (the eluent was a mixed solution of dichloromethane / methanol=10 / 1) to obtain 293 mg of the product as a yellow solid (yield: 85%). The product structural formula is as follows:
[0050]
[0051] 1 H NMR (400 ...
Embodiment 2
[0052] Example 2: Probe Fluorescent Detection of Hydrazine
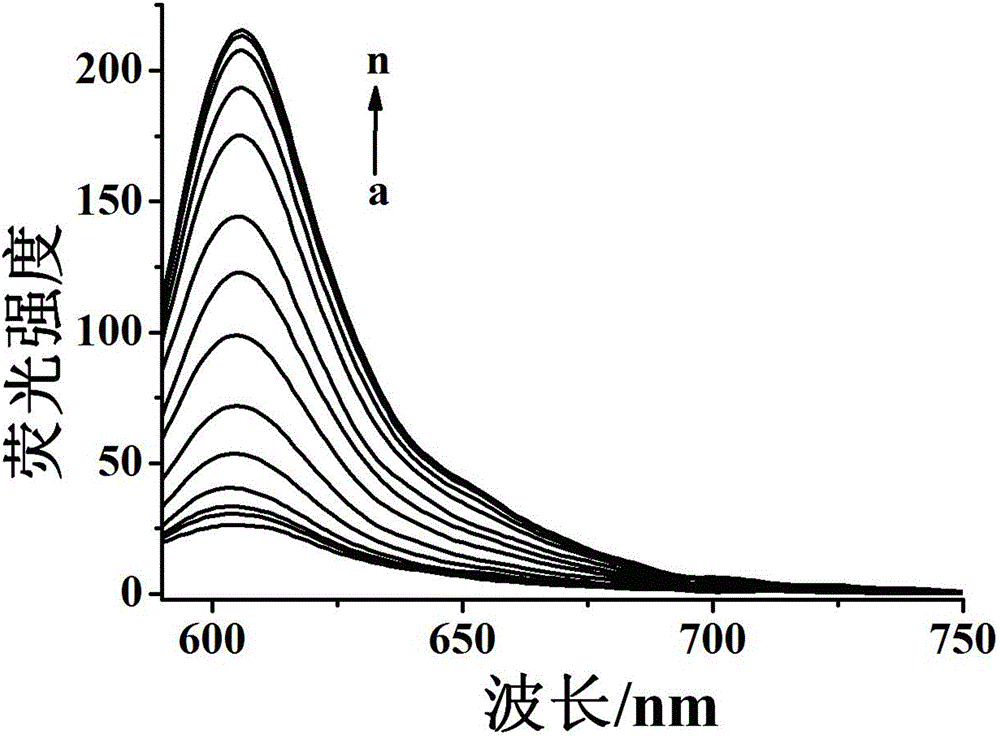
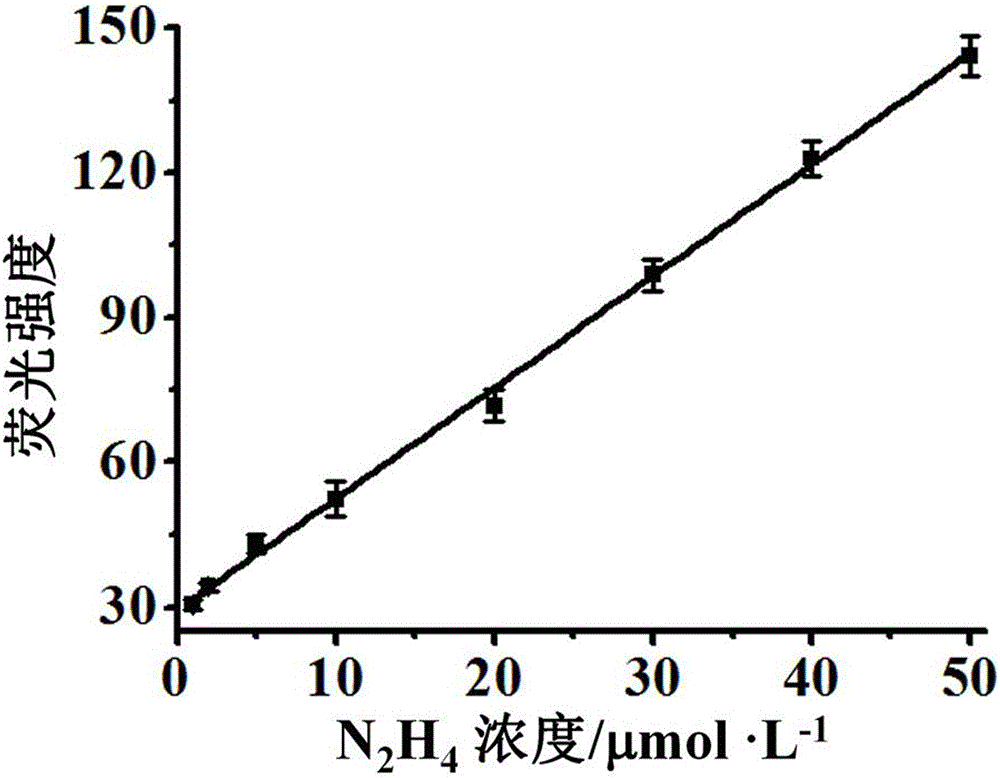
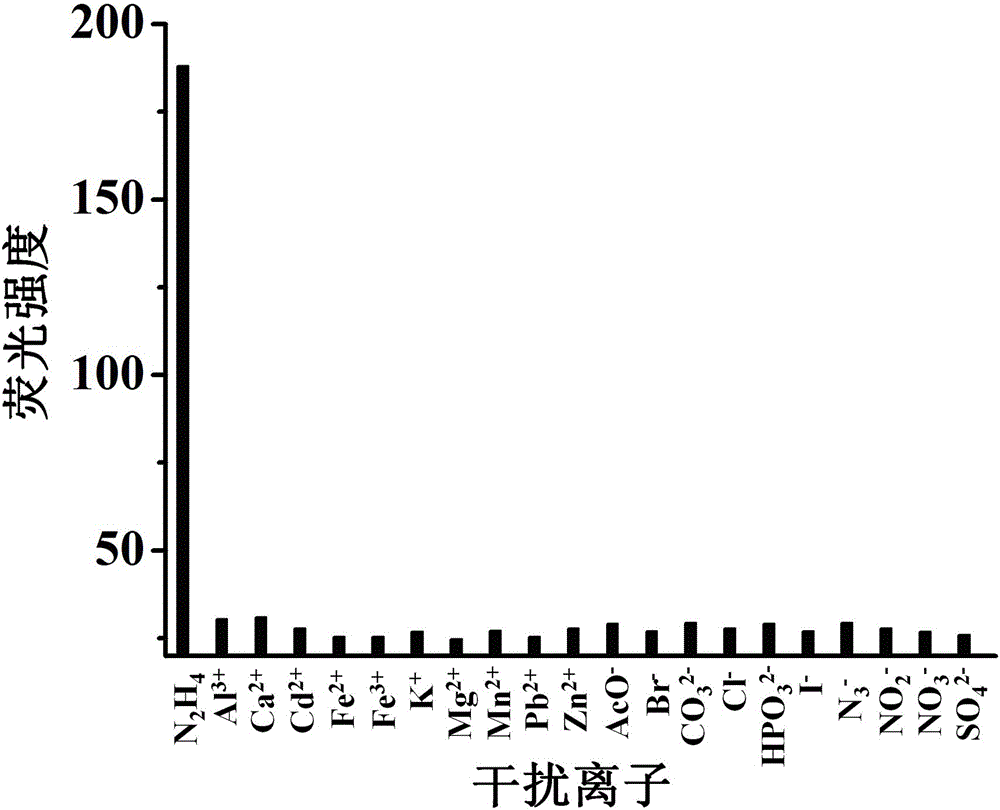
[0053] The molecular probe prepared above was dissolved in a mixed buffer solution of water and dimethyl sulfoxide (H 2 O / DMSO=9 / 1, v / v, 10 mM HEPES, pH 7.4)), prepared to 5 μmol L -1 probe solution. Add 2 mL of prepared 5 μmol L to a 3 mL cuvette -1 Probe solution of the present invention, then add different concentrations of hydrazine and mix evenly, test its fluorescence spectrum, the results are as follows image 3 shown. The fluorescence emission intensity of the solution at 620nm is plotted against the concentration of hydrazine, and the concentration of hydrazine is 1.0 – 50 μmol L -1 In the range, there is a good linear relationship between the two ( Figure 4 ), the quantitative detection of hydrazine within this concentration range can be realized, and the solution changes from yellow to purple, which is also suitable for naked eye detection. And this probe is not affected by some other common ions, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com