Tumor-targeting nanoparticles co-loaded with chemotherapeutic drugs and nucleic acids and preparation method thereof
A chemotherapeutic drug and nanoparticle technology, which is applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., to achieve the effects of easy operation, high biological safety, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
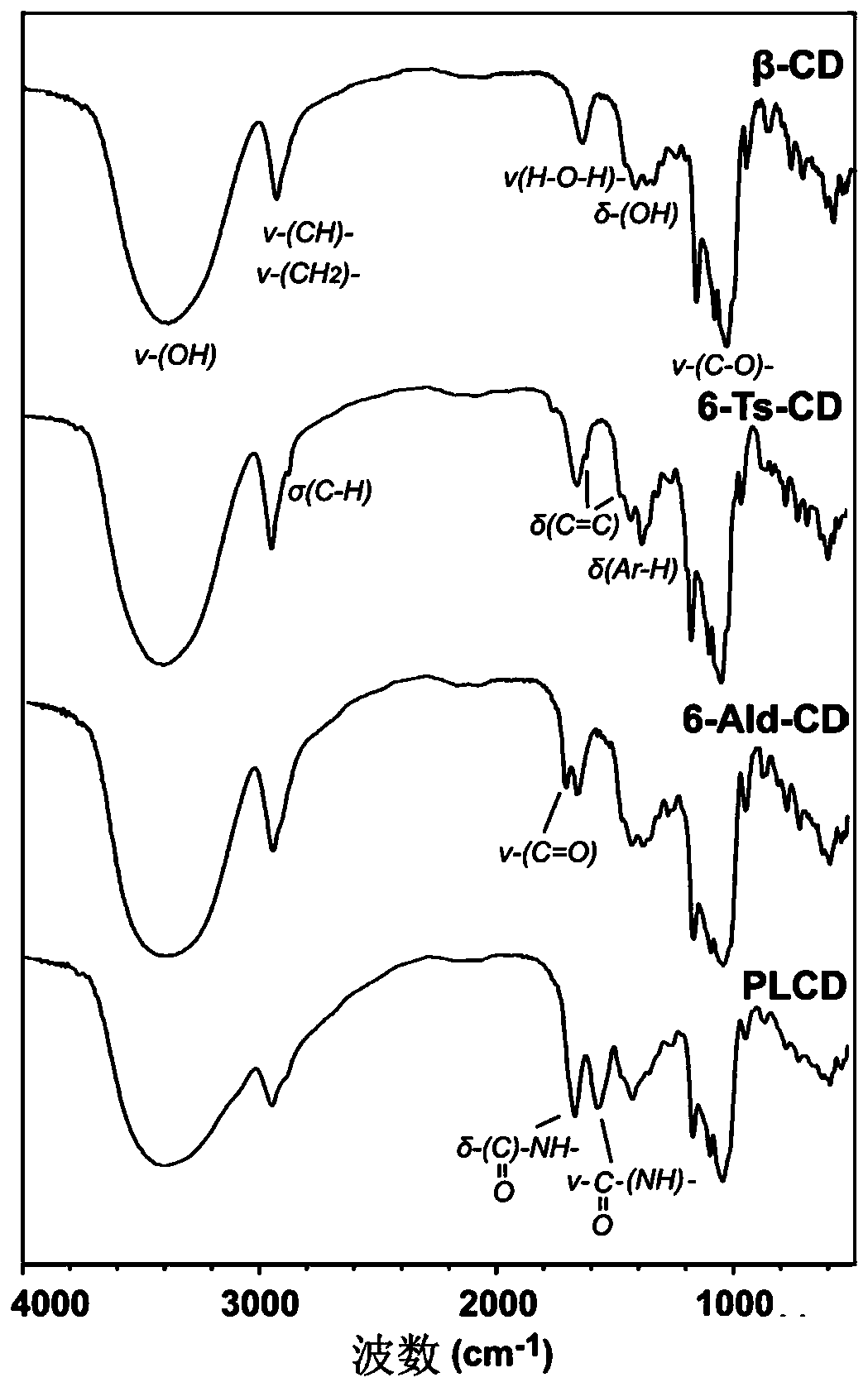
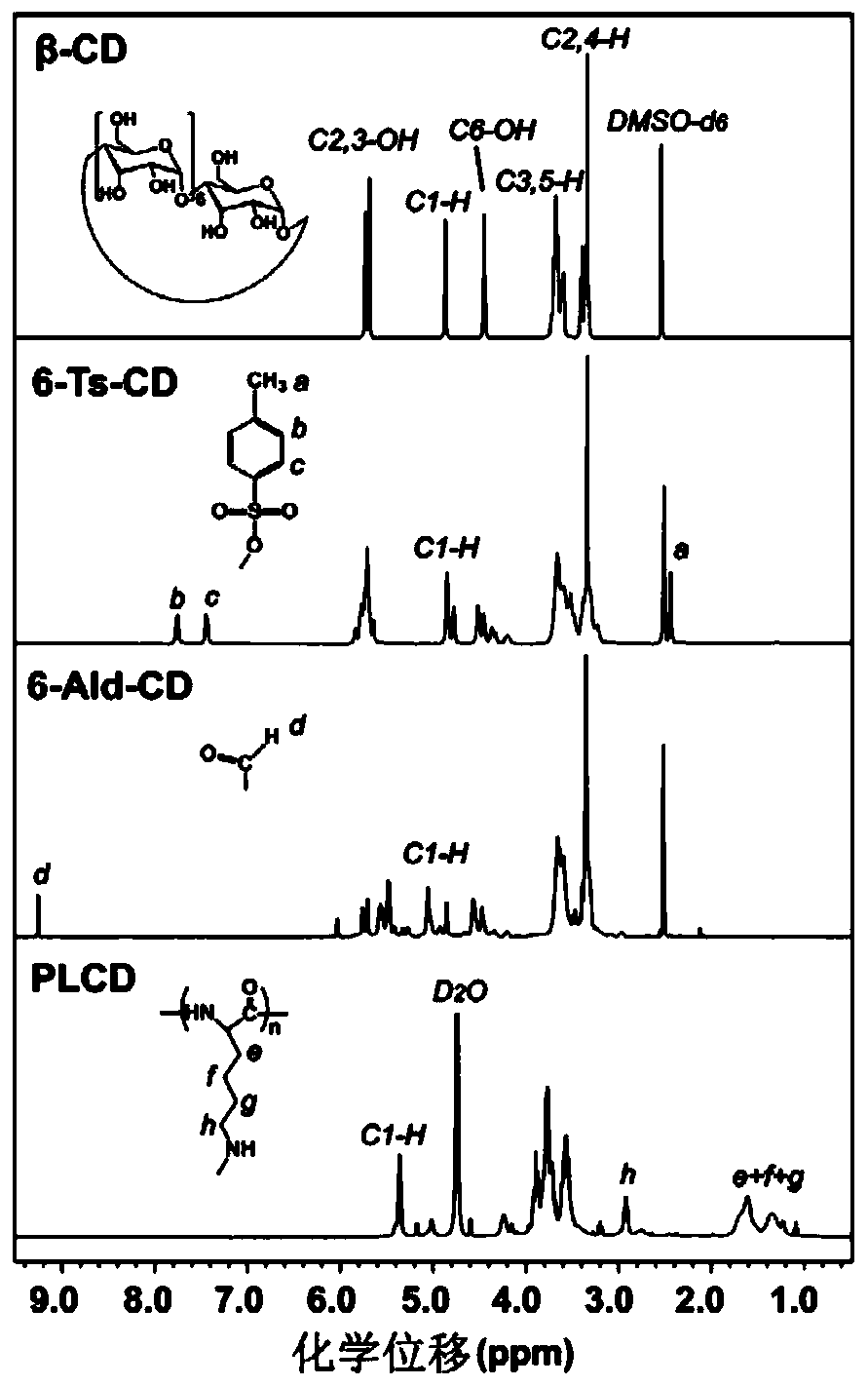
[0050] (1) Synthesis of polylysine grafted β-cyclodextrin derivatives (PLCD)
[0051] Dissolve 282mg (0.25mmol) of 6-Ald-CD, 57mg (of which the structural unit of lysine is 0.25mmol) of poly-L-lysine hydrobromide in 5mL of acetate buffer solution (0.2M) at pH 4.4 , stirred at room temperature for 1 h, added 62.8 mg (1 mmol) of sodium cyanoborohydride, continued to stir at room temperature for 72 h, added 2M NaOH aqueous solution to adjust the system to neutrality, and then transferred the reaction system to a dialysis bag (molecular weight cut-off of 7000 Da ), dialyzed in ultrapure water for 2 days, and the dialysate was freeze-dried. The resulting white floc product was polylysine-grafted β-cyclodextrin derivative (PLCD), in which the degree of substitution of β-CD was 12.9%.
[0052] The viscosity-average molecular weight of the poly-L-lysine hydrobromide is 15000-30000Da;
[0053] The chemical structure of PLCD was characterized by infrared spectrometer and nuclear magnet...
Embodiment 2
[0067] In vitro drug release experiments
[0068] Take three parts of PDR and HPDR prepared in Example 1, each 1 mL, transfer to a dialysis bag (molecular weight cut-off is 8000 ~ 14000Da), respectively placed in 10 mL of PBS solution with pH 5.0, 6.5 and 7.4, 37 ℃ avoid Light oscillation (100rpm), at different time points (see Figure 8 ) Take out 1.5mL medium for testing, and add an equal volume of fresh dialysis medium; use UV spectrophotometer to detect DOX release (detection wavelength is 480nm). The drug cumulative release rate was calculated according to the following formula: DOX cumulative release rate=(DOX release amount / total amount of DOX input)×100%. DOX release curve see Figure 8 , showing slow drug release characteristics and significant pH sensitivity.
Embodiment 3
[0070] Cellular Uptake Studies
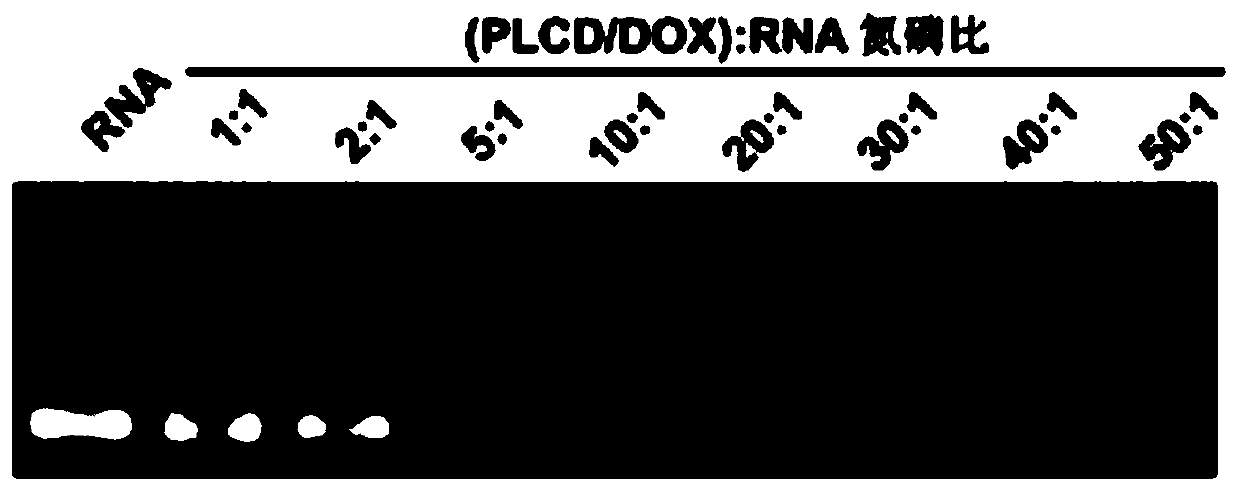
[0071] Replace Control RNA in Example 1 with FAM-RNA, others are the same as Example 1, and the prepared PDR and HPDR are respectively named as PDR FAM and HPDR FAM .
[0072] Examination of PDR by Hepatoma Cell Line MHCC-97H FAM and HPDR FAM The ability and localization of cells; 2 Cultivate in an incubator, the medium is high-sugar DMEM medium containing 10% FBS; when the cells are in the logarithmic growth phase, digest the cells according to 5×10 4 The density of cells / well was seeded in a 12-well plate pre-laid with special glass slides for laser confocal. After 24 hours of culture, free DOX, FAM-RNA, and PDR diluted in serum-free medium were added. FAM and HPDR FAM , so that the final concentration of DOX in the system was 3.3 μg / mL, and the final concentration of FAM-RNA was 1.0 μg / mL; after incubation for 4 hours, the cells were fixed with 4% paraformaldehyde, and the nuclei were stained with DAPI. To observe the fluorescence in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com