Aspergillus unguis bromo-depsidone compound and preparation method and application thereof
A technology of Aspergillus brominated depsipepsyl acid cyclic ether and depsipsipsic acid cyclic ether, which is applied in the field of microbial fermentation product extraction, can solve the problems of antibiotics losing curative effect, and achieve good antifungal properties, wide application prospects, and easy large-scale production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
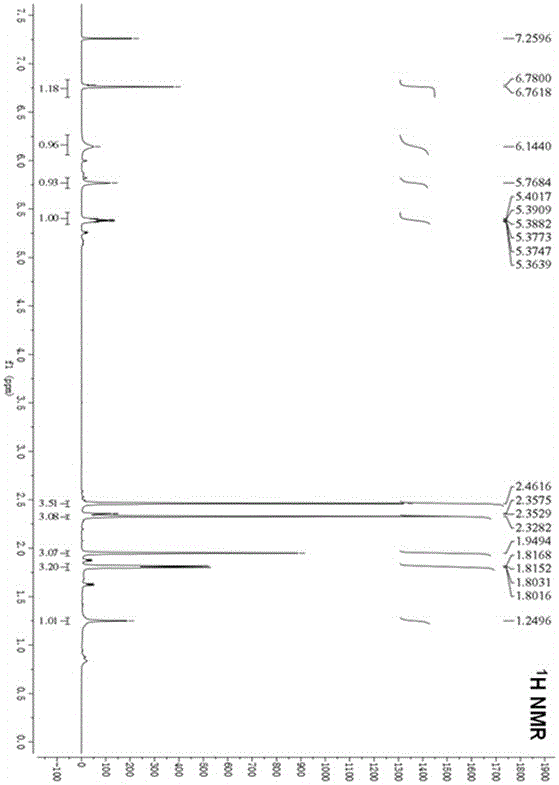
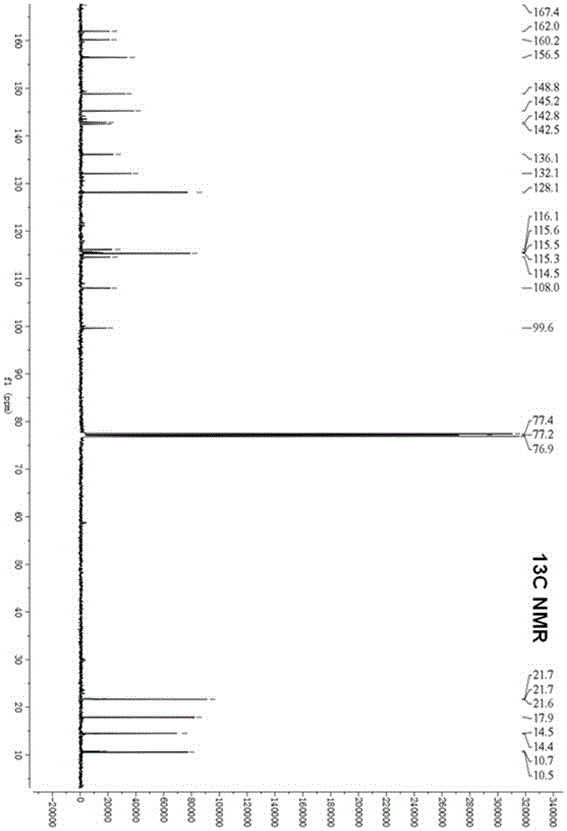
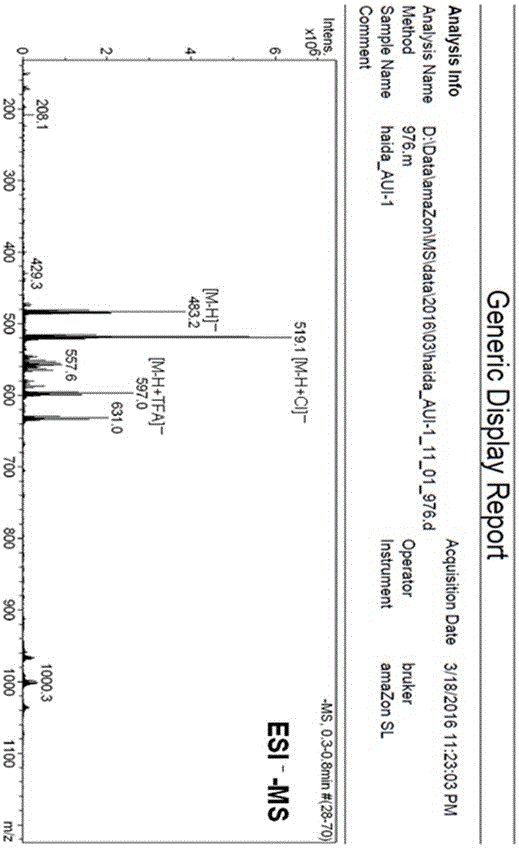
[0047] Example 1 The preparation method of marine fungal brominated depsipepsyl acid cyclic ether compounds
[0048] 1. Fermentation
[0049] Aspergillus claw ( Aspergillus unguis ) CGMCC No.3372 was inoculated in the fungal liquid medium for static fermentation. After 2 days of fermentation, procaine solution with a final concentration of 1mM was added, and the fermentation was continued for 20 days, filtered, and the mycelia and fermentation broth were collected respectively.
[0050] Wherein, the formula of described fungal liquid culture medium is:
[0051] Each liter contains the following components: 500 mL of potato juice, 20 g of NaBr, 20 g of sucrose, and 500 mL of pure water or tap water.
[0052] 2. Rough extraction
[0053] The fermented liquid obtained in step 1 was extracted three times with ethyl acetate, concentrated under reduced pressure to obtain a concentrated liquid. At the same time, the mycelium obtained in step 1 was extracted three times with methano...
Embodiment 2
[0071] Example 2 Experiment of antifungal activity of brominated depsipphenolic acid cyclic ether compounds
[0072] 1. Candida albicans ( Candida albicans ) is a common opportunistic fungus that can invade many parts of the human body, causing skin candidiasis, mucosal candidiasis (thrush, angular cheilitis, and vaginitis are the most common) and visceral and central nervous candidiasis. Common fungal diseases. The clinical symptoms are intricate and varied in urgency and slowness. In recent years, with the high-dose application of antibiotics, hormones, and immunosuppressants, as well as the development of organ transplantation, the incidence of candidiasis has gradually increased, and can be life-threatening and cause serious consequences. This bacterium has become an important target for the development of antifungal drugs.
[0073] 2. Experimental method:
[0074] Use the international standard micro broth dilution method (National Committee for Clinical LaboratorySt...
Embodiment 3
[0079] Example 3 Anti-Gram-negative Bacteria Activity Experiment of Bromo-Depsipphenolic Acid Cyclic Ether Compounds
[0080] 1. Pseudomonas aeruginosa ( Pseudomonas aeruginosa ), also known as Pseudomonas aeruginosa, is an opportunistic pathogen and one of the main pathogens of nosocomial infection. Patients suffering from metabolic diseases, blood diseases and malignant tumors, as well as patients after surgery or certain treatments are susceptible to infection with this bacteria. The bacteria often cause postoperative wound infection, and can also cause bedsores, abscesses, and suppurative otitis media. The infected lesions caused by it can lead to hematogenous dissemination, and bacteremia and sepsis occur. Infection with Pseudomonas aeruginosa after burns can cause death. Moreover, with the widespread clinical use of broad-spectrum antibiotics, hormones, and immunosuppressants, Pseudomonas aeruginosa has rapidly developed resistance to a variety of antibiotics, making...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com