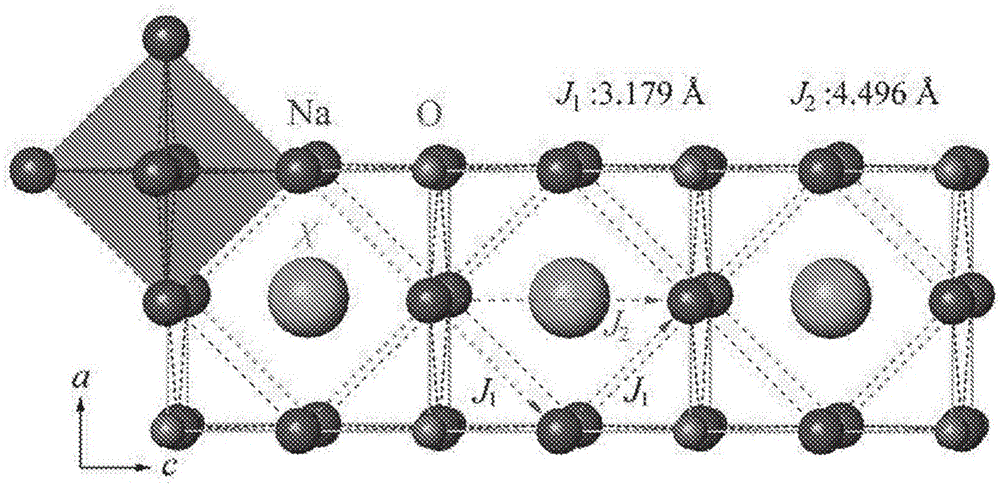
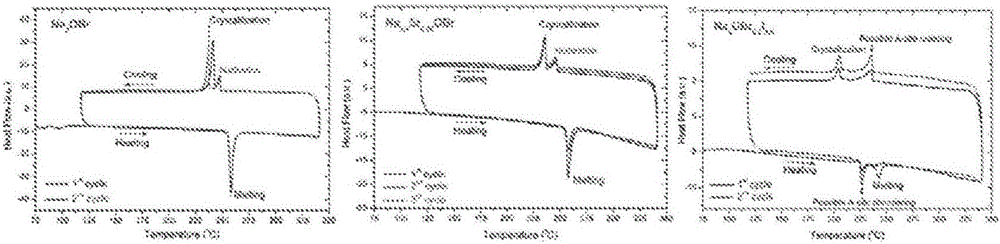
Sodium anti-perovskite solid electrolyte compositions
A technology of solid electrolyte and anti-perovskite, applied in the direction of electrolyte, sodium/potassium compound, calcium/strontium/barium compound, etc., can solve the problems of poor machinability and high cost, achieve optimized performance and improve ion conductivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0037] Na 3 Preparation of OCl: Weigh out 0.400g NaOH and 0.585g NaCl and place in N 2 Grind together in air for a few minutes. The resulting fine powder was spread on 0.253 g of Na metal and the mixture was placed in an alumina crucible, which was then sealed in a quartz tube. The sample was first heated to 150°C under vacuum at a heating rate of 1.5°C / min (through the melting point T of Na metal m =97.8°C), and then heated to 350°C at a heating rate of 10°C / min. 1 mol of reactant will release 0.5 mol of H during the heating process 2 , so care and proper handling must be taken when conducting said tests and the total amount of raw material should be well planned. After 3 hours at the highest reaction temperature, the sample was allowed to cool naturally to room temperature. By repeating the milling and heating process 3 times, Na 3 Phase pure powder of OCl. The total synthetic route for a batch of samples took approximately 24 hours.
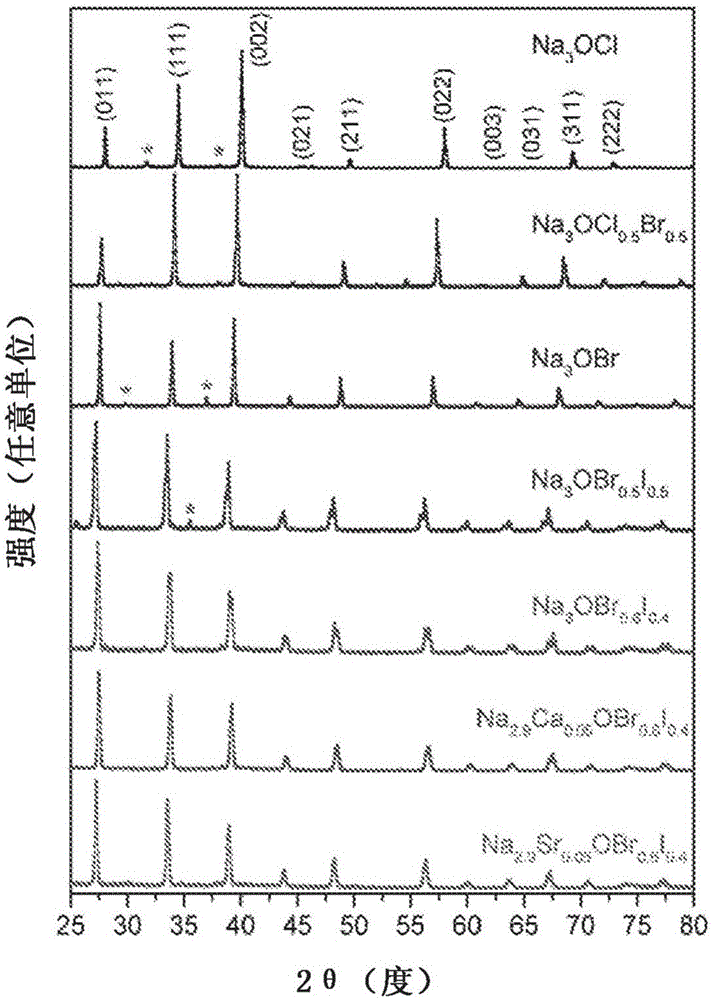
[0038] Powder X-ray diffraction...
Embodiment B
[0042] Na 3 OBr 0.5 I 0.5 Preparation: Weigh out 0.400g NaOH, 0.515g NaBr and 0.645g NaI and in N 2 Grind together in air for a few minutes. The resulting fine powder was spread on 0.253 g of Na metal and the mixture was placed in an alumina crucible, which was then sealed in a quartz tube. The sample was first heated to 150°C under vacuum at a heating rate of 1.5°C / min (through the melting point T of Na metal m =97.8°C), and then heated to 350°C at a heating rate of 10°C / min. After 3 hours at the highest reaction temperature, the sample was naturally cooled to room temperature. By repeating the milling and heating process 3 times, Na 3 OBr 0.5 I 0.5 phase pure powder. The total synthetic route for a batch of samples took approximately 24 hours.
[0043] Powder X-ray diffraction data were collected at room temperature (25°C). Before measurement, the sample was placed in N 2 Packaged in experimental film (PARAFILM "M") under atmosphere to avoid moisture absorption. ...
Embodiment C
[0045] Na 2.9 Sr 0.05 OBr 0.5 I 0.5 Preparation: Weigh out 0.360g NaOH, 0.515g NaBr, 0.645g NaI and 0.052g SrO and in N 2 Grind together in air for a few minutes. The resulting fine powder was spread on 0.253 g of Na metal and the mixture was placed in an alumina crucible, which was then sealed in a quartz tube. The sample was first heated to 150°C under vacuum at a heating rate of 1.5°C / min (through the melting point T of Na metal m =97.8°C), and then heated to 350°C at a heating rate of 10°C / min. After 3 hours at the highest reaction temperature, the sample was naturally cooled to room temperature. By repeating the milling and heating process 3 times, Na 2.9 Sr 0.05 OBr 0.5 I 0.5 phase pure powder. The total synthetic route for a batch of samples took approximately 24 hours.
[0046] Powder X-ray diffraction data were collected at room temperature (25°C). Before measurement, the sample was placed in N 2 Packaged in experimental film (PARAFILM "M") under atmosph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com