Method for preparing hydroxyalkyl disiloxane
A technology of hydroxyalkyl disiloxane and hydrochlorosilane, which is applied in the field of synthesis of organosilicon compounds, can solve the problems of unsuitability for industrial production, high operation requirements, and difficulty in purification, and achieve simple steps, mild conditions, and easy industrialization The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The preparation of 1,3-bis(hydroxypropyl)-1,1,3,3-tetramethyldisiloxane, its structural formula is as shown in formula (Ⅴ):
[0051]
[0052] Including the following steps:
[0053] (1) In a four-necked flask equipped with a reflux condenser, a T-type three-way piston, a constant pressure dropping funnel, and a rubber stopper, pass an inert gas for a period of time, and keep the speed of entry, and add 10.012g vinyl acetate in the flask Propyl ester, 15.0mL toluene, 9.462g dimethyl monohydrochlorosilane, after starting to stir, add 0.1mL ethylenediamine platinum complex-isopropanol solution (concentration is 0.12mol / L), and control the reaction temperature to 50 The reaction at ℃ gave 3-acetoxypropyl dimethylchlorosilane with a yield of about 82.9%.
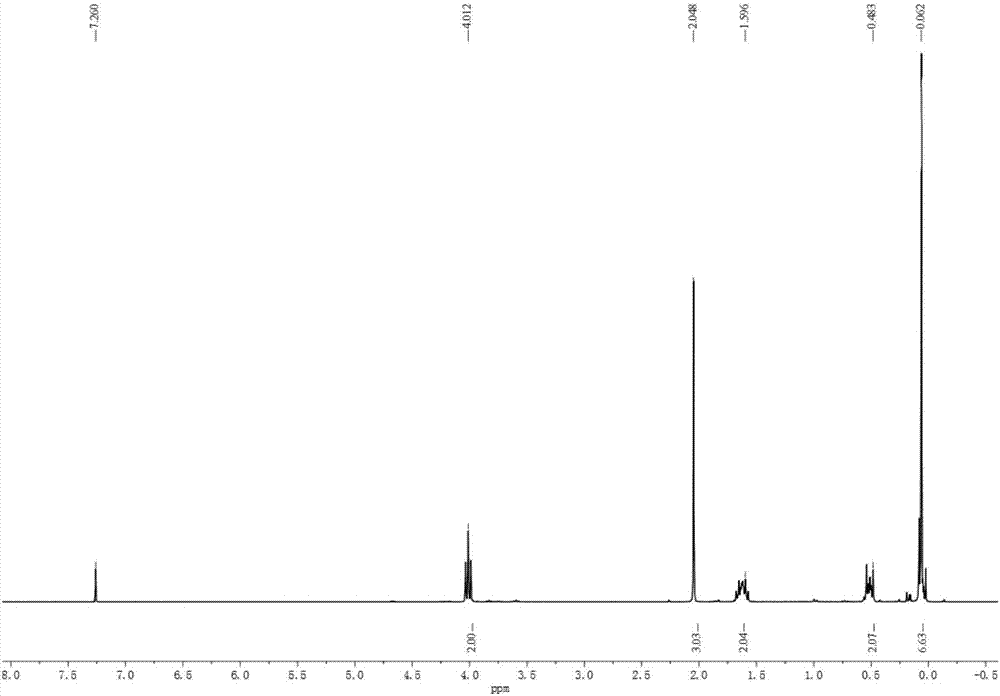
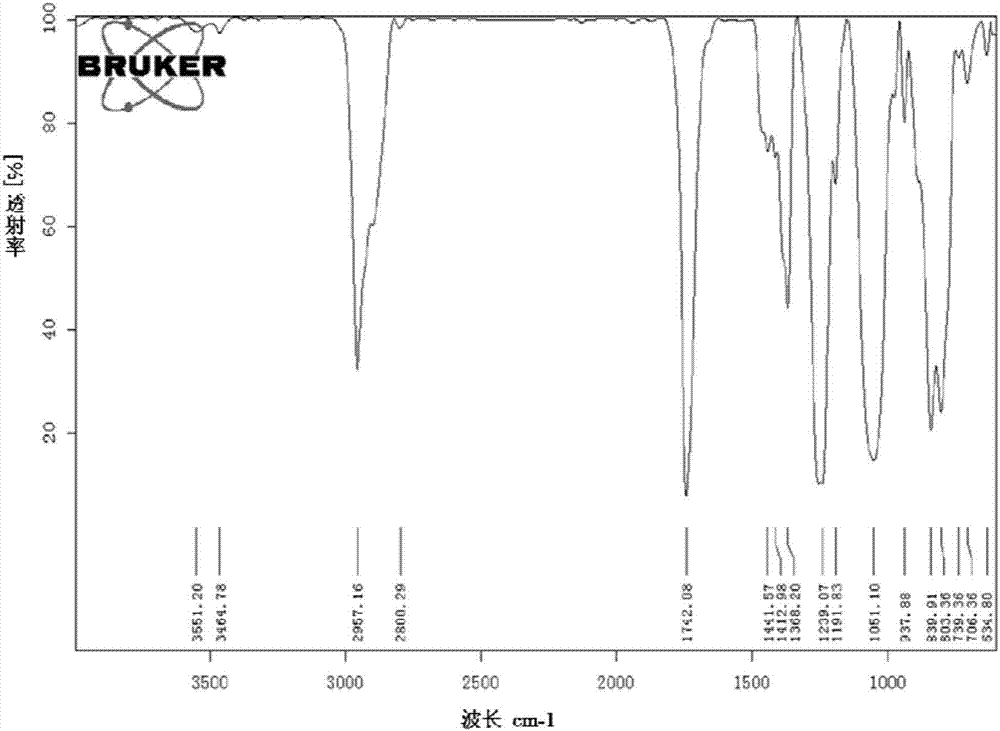
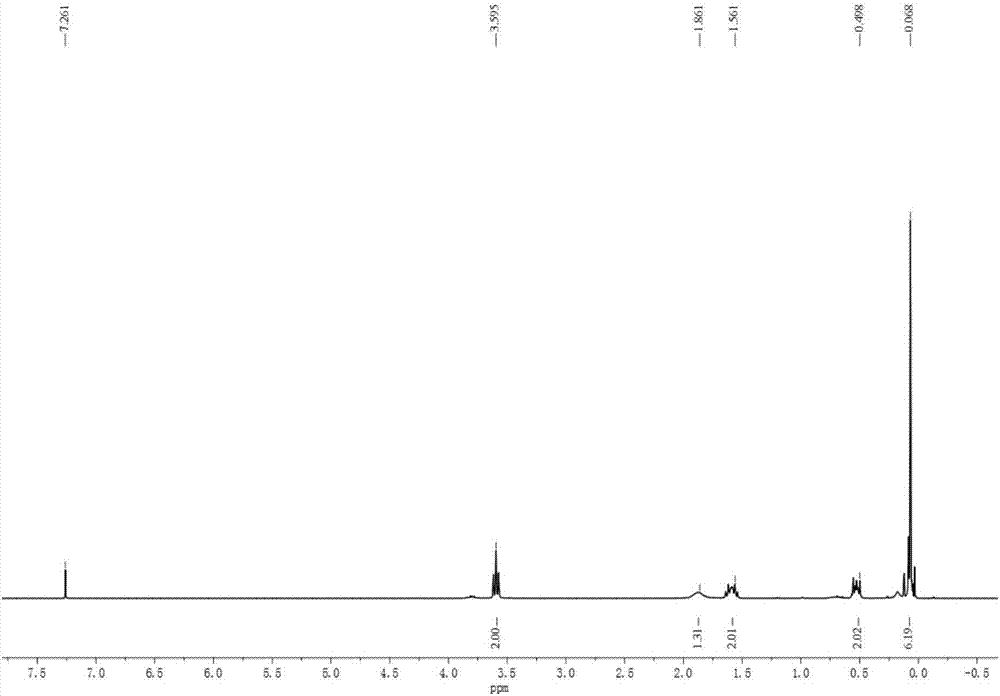
[0054] 3-Acetoxypropyl dimethyl chlorosilane is tested by nuclear magnetic hydrogen spectrum and infrared spectrum, such as figure 1 , figure 2 shown.
[0055] (2) Add the product obtained in step (1) into a single...
Embodiment 2
[0059] The preparation of 1,3-bis(hydroxypropyl)-1,1,3,3-tetramethyldisiloxane, its structural formula is as shown in formula (Ⅴ):
[0060] Including the following steps:
[0061] (1) In a four-necked flask equipped with a reflux condenser, a T-shaped three-way piston, a constant pressure dropping funnel, and a rubber stopper, pass inert gas for a period of time to maintain the speed of entry, and add 20.025g vinyl acetate to the flask. Propyl ester, 30.0mL toluene, 18.924g dimethyl monohydrochlorosilane, after starting to stir, add 0.1mL ethylenediamine platinum complex-toluene solution (concentration: 0.12mol / L), and control the reaction temperature to 50℃ The reaction yielded 3-acetoxypropyldimethylchlorosilane with a yield of about 80.5%.
[0062] (2) Add the product obtained in step (1) into a single-necked flask equipped with a constant-pressure dropping funnel, and drop a mixed liquid of HCl solution and acetone solution with pH=4 therein at 50° C. to obtain a hydrolyz...
Embodiment 3
[0065] The preparation of 1,3-bis(hydroxybutyl)-1,1,3,3-tetramethyldisiloxane, its structural formula is as shown in formula (Ⅵ):
[0066] Including the following steps:
[0067]
[0068] (1) In a four-necked flask equipped with a reflux condenser, a T-type three-way piston, a constant-pressure dropping funnel, and a rubber stopper, feed inert gas for a period of time, keep the feeding speed, and add 17.121g of acetic acid in the flask- 3-butenyl ester, 23.0mL toluene, 14.193g dimethyl monohydrochlorosilane, after starting to stir, add 0.1mL ethylenediamine platinum complex-toluene solution (concentration is 0.12mol / L), and control the reaction temperature to The reaction at 80° C. gave 4-acetoxy n-butyldimethylchlorosilane with a yield of about 78.7%.
[0069] (2) Add the product obtained in step (1) into a single-necked flask equipped with a constant pressure dropping funnel, and add CH with pH=2 dropwise at 100°C 3 A mixed liquid of COOH aqueous solution and toluene so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com