Mosapride citrate tablet and preparation method thereof
A technology of mosapride citrate and tablet, applied in the field of medicine, can solve the problems of low mosapride citrate, cumbersome process, difficult to fully mix, etc., and achieves rapid drug dissolution and simple preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
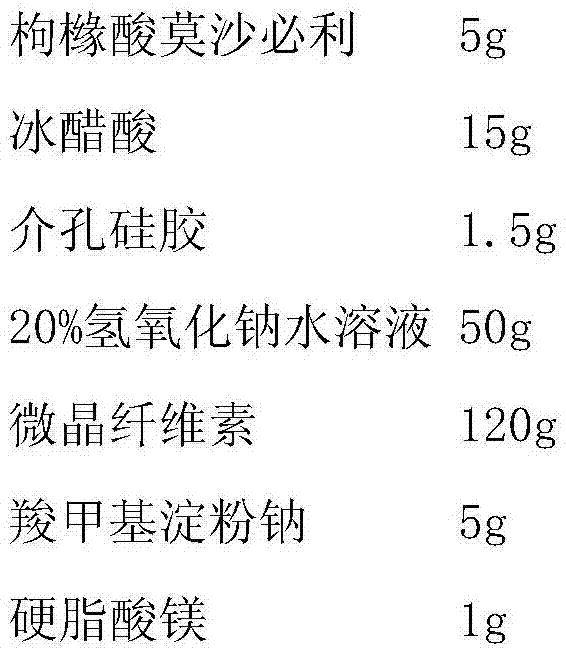
[0022]
[0023] Preparation Process:
[0024] Dissolve mosapride citrate in glacial acetic acid, add mesoporous silica, stir evenly, add sodium hydroxide aqueous solution to this solution under stirring condition, mosapride citrate and mesoporous silica The silicon oxide complex is precipitated, filtered, dried at 60°C, then mixed evenly with the mixed powder of microcrystalline cellulose and sodium carboxymethyl starch, added with magnesium stearate, mixed, and pressed into tablets.
Embodiment 2
[0026]
[0027]
[0028] Preparation Process:
[0029] Dissolve mosapride citrate in glacial acetic acid, add mesoporous silica, stir evenly, add sodium hydroxide aqueous solution to this solution under stirring condition, mosapride citrate and mesoporous silica The silicon oxide complex is precipitated, filtered, dried at 65°C, then mixed evenly with the mixed powder of microcrystalline cellulose and crospovidone, added with magnesium stearate, mixed, and pressed into tablets.
Embodiment 3
[0031]
[0032] Preparation Process:
[0033] Dissolve mosapride citrate in glacial acetic acid, add mesoporous silica, stir evenly, add sodium hydroxide aqueous solution to this solution under stirring condition, mosapride citrate and mesoporous silica The silicon oxide complex is precipitated, filtered, dried at 60°C, then mixed evenly with the mixed powder of microcrystalline cellulose and sodium carboxymethyl starch, added with magnesium stearate, mixed, and pressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com