Sediment and deposition preparing method of Pd/Mg(OH)2 catalyst and application of Pd/Mg (OH)2 catalyst
A technology for catalysts and preparation steps, which is applied in the field of preparation of catalysts for the synthesis of dimethyl oxalate by CO gas-phase catalytic coupling, which can solve the problems that are not conducive to the efficient utilization of noble metal Pd, increase catalyst production costs, and high loading capacity, and achieve low pollution , Ease of operation, and improved dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
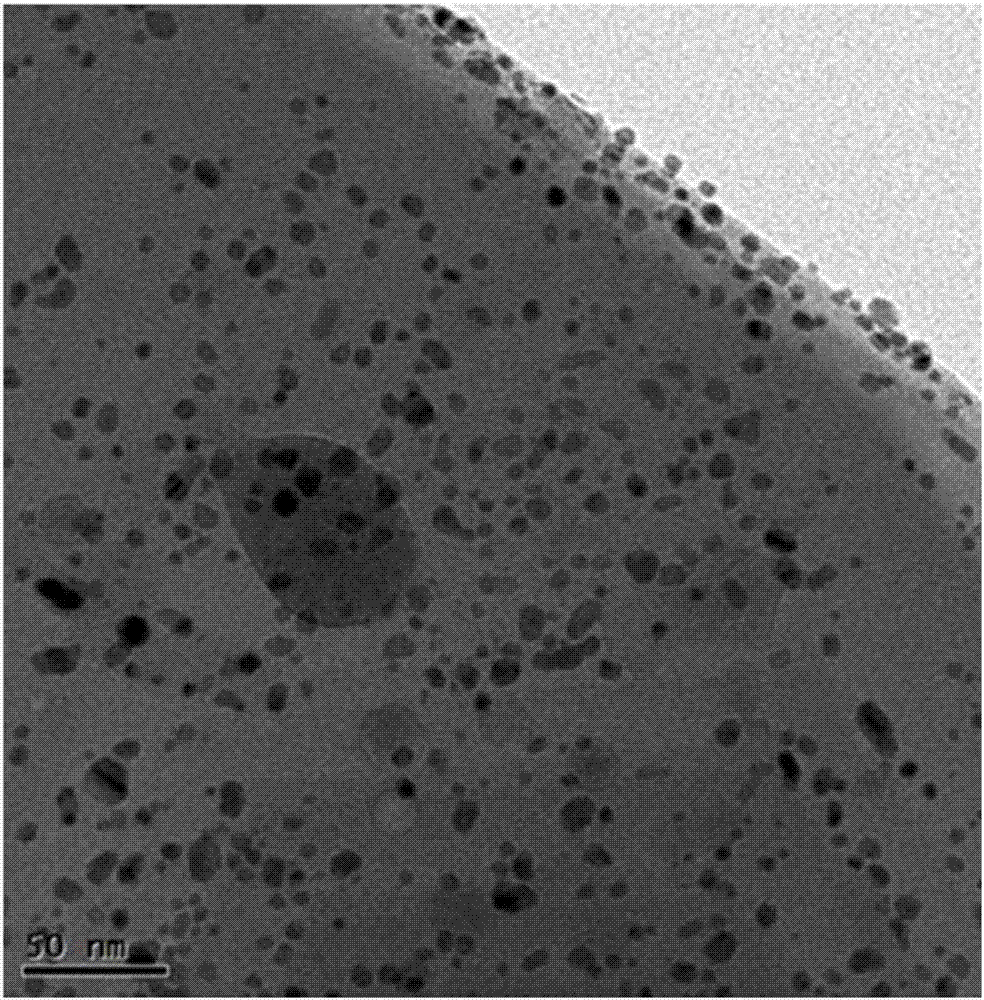
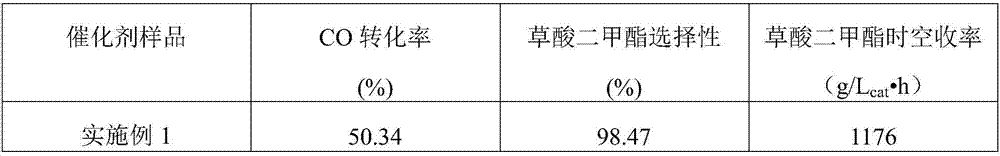
[0021] Take 20mL of the pre-prepared mixed solution of magnesium nitrate and sodium chloropalladate and place it in a 70°C water bath, in which the concentration of magnesium nitrate is 1mol / L, and the concentration of sodium chloropalladate is 0.5mmol / L. Use a dropping funnel to add 1mol / L sodium hydroxide solution to it at a rate of 15 drops / min. When the pH value of the solution at the end of the precipitation reaction is 8.5, stop adding lye and keep it in a 70°C water bath. Stand and age for 12 hours, filter with suction, and wash with deionized water until the filtrate is neutral, dry the obtained solid powder in an oven at 150°C for 12 hours, and then reduce it with pure hydrogen at 150°C for 2 hours to obtain the desired catalyst sample. Using ICP analysis, the mass fraction of noble metal Pd in the carrier is 0.091%. The dispersion of Pd was tested by CO static chemisorption, and the results are shown in Table 1.
Embodiment 2
[0023] Take 20mL of the pre-prepared 2mol / L magnesium acetate and 3.5mmol / L tetraammine palladium nitrate mixed solution and place it in a water bath at 80°C, and then use a dropping funnel to drip at a rate of 20 drops / min under constant stirring. Add 2mol / L sodium hydroxide solution, when the pH value of the solution at the end of the precipitation reaction is 9, stop adding lye, keep it in a water bath at 80°C for 8 hours, and then suction filter the obtained precipitate , and washed with deionized water until the filtrate is neutral, and finally the obtained solid powder was dried in an oven at 120°C for 18 hours, and after drying, it was reduced with pure hydrogen at 150°C for 4 hours to obtain the desired catalyst sample. Using ICP analysis, the mass fraction of noble metal Pd in the carrier is 0.32%. The results of the dispersion test of Pd are shown in Table 1.
Embodiment 3
[0025] Take 20mL of a pre-prepared mixed solution of 3mol / L magnesium chloride and 8mmol / L palladium chloride and place it in a 60°C water bath, then add 2.5mol / L of potassium hydroxide solution, when the pH value of the solution at the end of the precipitation reaction is 10.5, stop adding the lye, keep it in a water bath at 60°C for 20 hours, and then filter the precipitate with suction and use it to remove Wash with ionic water until the filtrate is neutral, and finally place the obtained solid powder in an oven at 150°C for 20 hours, and then reduce it with pure hydrogen at 150°C for 4 hours to obtain the desired catalyst sample. Using ICP analysis, the mass fraction of noble metal Pd in the carrier is 0.47%. The results of the dispersion test of Pd are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com