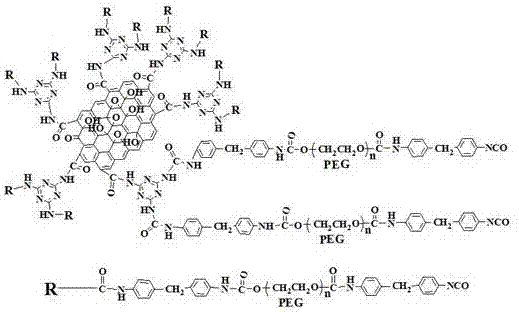
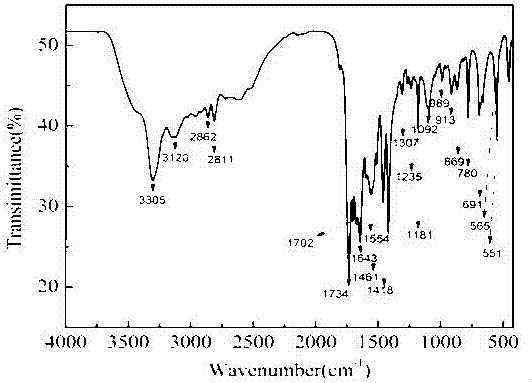
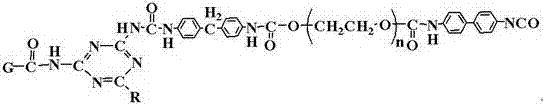Polyurethane phase-change material containing polyethylene glycol regulated by functionalized graphene and preparation method of polyurethane phase-change material
A technology of polyethylene glycol and phase change materials, applied in the field of phase change energy storage, can solve problems affecting the long-term performance of phase change materials, phase separation, etc., and achieve improved thermal stability, thermal conductivity, and flame retardancy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 1.20g of graphene oxide GO and place it in a 250ml three-neck flask, add 1ml of N,N-dimethylformamide (DMF) into it, start stirring slowly, and slowly add 80ml of it to the above system with a constant pressure dropping funnel Thionyl chloride (SOCl 2 ), and then heated to 70°C for 24 hours to obtain the reaction product Intermediate I (about 0.65 g) after vacuum drying.
[0032]Weigh 4.62g of CAC and place it in a 250ml flask under ice bath conditions, slowly add 50ml of refrigerated acetone and 50ml of deionized water in turn, form a uniform suspension after magnetic stirring, and keep at 0°C during this process. Then slowly add 100ml of ammonia water with a concentration of 1mol / L to the above suspension with the help of a dropping funnel, and keep it at 0~5°C for 30min. After filtering, washing with water and drying at 50°C, the product ADCT, namely intermediate II, was obtained.
[0033] Intermediate I and Intermediate II were heated to 85° C. and stirred f...
Embodiment 2
[0037] Add polyethylene glycol 4000 (27.00g) to step V in Example 1 and dissolve in the fully purified DMF solvent, add MDI in a molar ratio (2:1), keep at 70-80°C under the protection of nitrogen Next, add 0.1-0.5% of dibutyltin dilaurate (T-12, DBTDL) to the amount of catalyst PEG solid powder, and react for 4-6h. Other steps are the same as in Example 1.
[0038] The phase change enthalpy of the phase change material is 119.6J / g, the enthalpy change efficiency is 68.73%, the heat release rate is reduced by 42.7%, the ignition time of the material is delayed by 51S, and the thermal conductivity is increased by 322%.
Embodiment 3
[0040] Add polyethylene glycol 10000 (66.67g) to step V in Example 1 and dissolve it in the fully purified DMF solvent, add MDI in a molar ratio (2:1), keep it at 70-80°C under the protection of nitrogen Next, add 0.1-0.5% of dibutyltin dilaurate (T-12, DBTDL) to the amount of catalyst PEG solid powder, and react for 4-6h. Other steps are the same as in Example 1.
[0041] The phase change enthalpy of the phase change material is 133.7J / g, the enthalpy change efficiency is 82.33%, the heat release rate is reduced by 27.8%, the ignition time of the material is delayed by 27S, and the thermal conductivity is increased by 237%.
[0042] In the above examples, only the molecular weight of polyethylene glycol is different. The test results show that its energy storage density, thermal stability and thermal conductivity of the composite phase change material through the regulation and control of molecular weight are all improved compared with pure polyethylene glycol, and it can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase change enthalpy | aaaaa | aaaaa |
| Phase change enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com