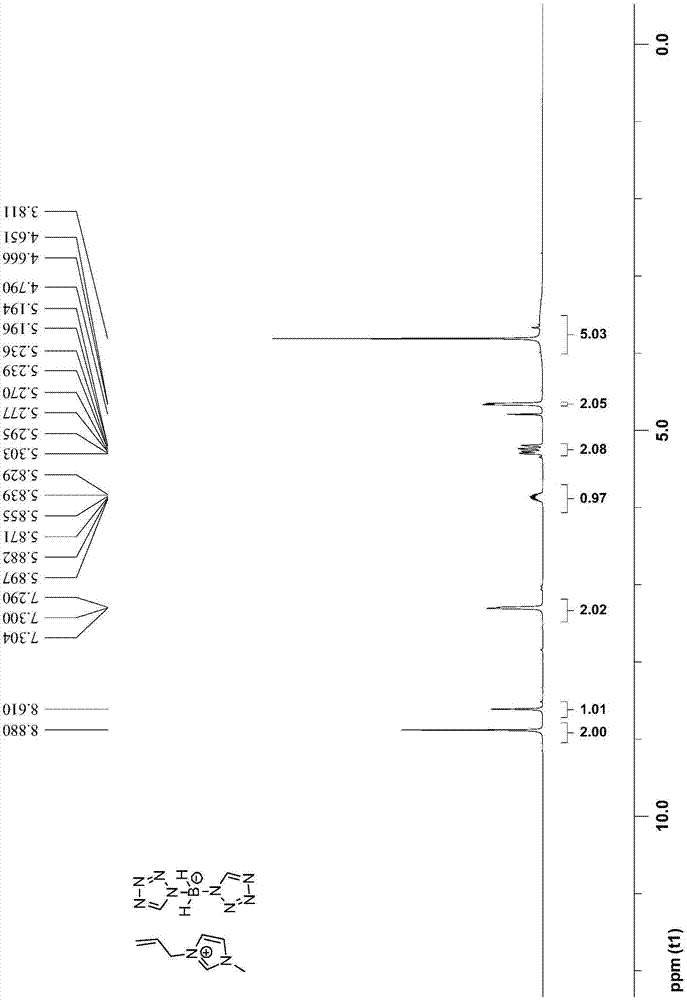
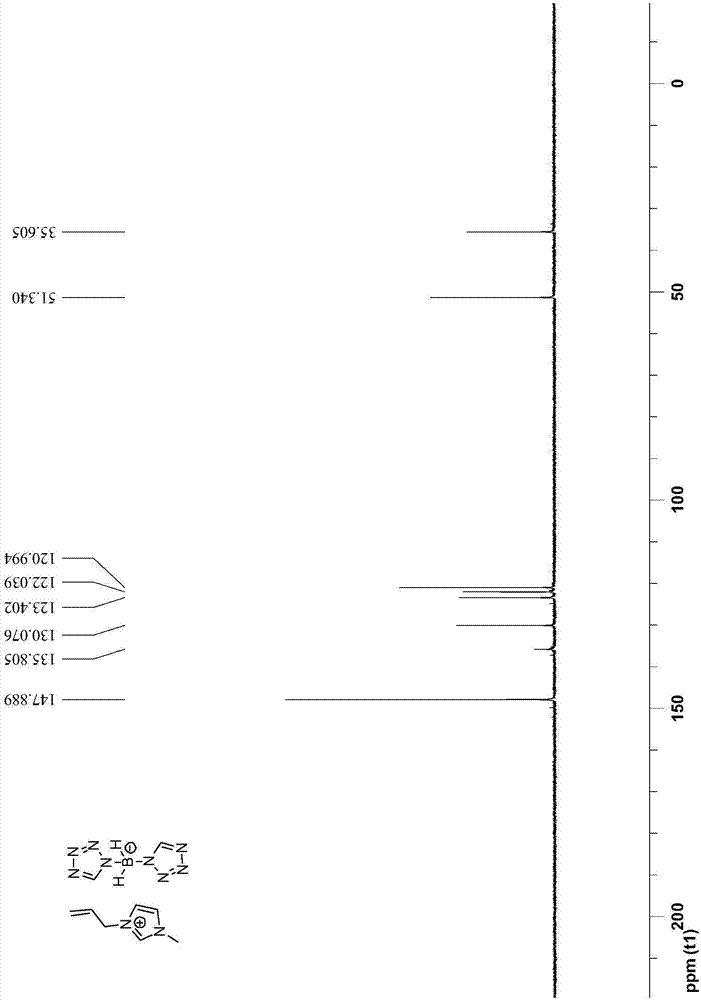
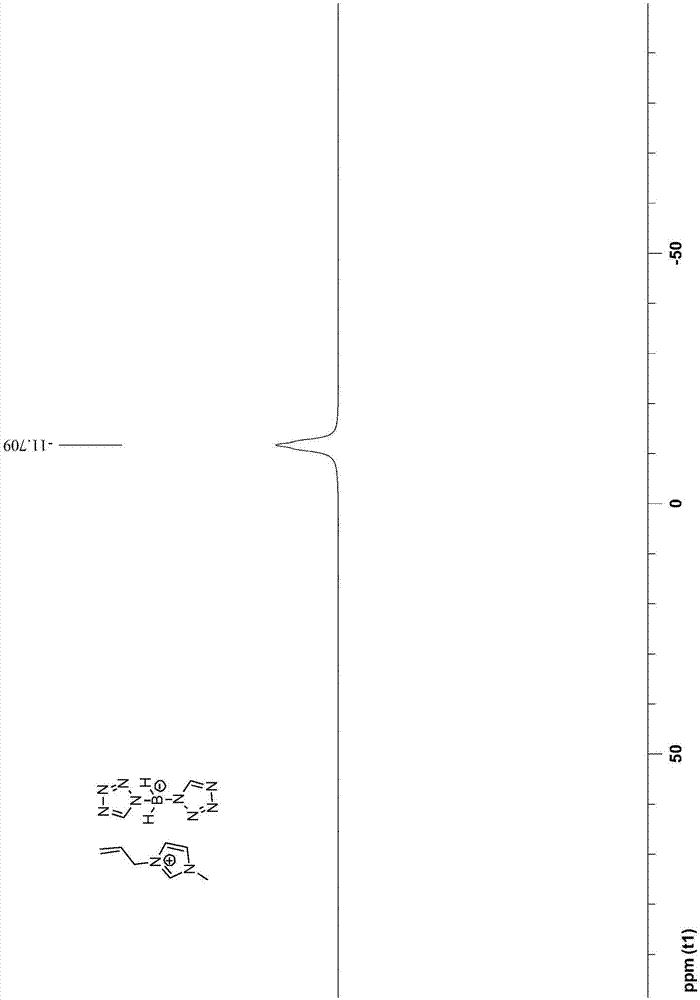
Bis(tetrazolyl) boric acid ionic liquid and preparation method thereof
A technology of ionic liquid and imidazolium cation, which is applied in the field of bis-boric acid ionic liquid and its preparation, can solve the problems of explosion hazard and achieve the effects of reducing risk, wide liquid range, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation steps of 1-allyl-3-methyl-1H-imidazole bis(tetrazole) borate are as follows:
[0049] (1) Under the protection of argon, first add 106mmol of sodium borohydride to 70mL of anhydrous acetonitrile, and then add 212mmol of 1H-tetrazole in batches to obtain the reaction solution I; the reaction solution I is refluxed and stirred at 85°C for 72h , cooled to room temperature, filtered and collected solid matter, vacuum-dried to obtain bis(tetrazole) borate sodium salt;
[0050] (2) Add 40mmol 1-allyl-3-methyl-1H-imidazolium chloride salt and 44mmol bis(tetrazole) borate sodium salt to 100mL acetonitrile to obtain reaction solution II; reaction solution II was stirred at 25°C After reacting for 7 days, filter and collect the filtrate; after removing the acetonitrile solvent in the filtrate by rotary evaporation, first dissolve it with 50mL of dichloromethane, then wash it with water three times, then dry it with anhydrous sodium sulfate, then carry out vacuum d...
Embodiment 2
[0060] Synthesis of 1-ethyl-3-methyl-1H-imidazolium bis(tetrazole)borate
[0061] (1) Under the protection of argon, first add 106mmol of sodium borohydride into 70mL of anhydrous acetonitrile, and then add 212mmol of 1H-tetrazole in batches to obtain the reaction solution I; the reaction solution I was refluxed and stirred for 72 hours at 85°C, then cooled to room temperature, filtered and collected solid matter, and dried in vacuo to obtain bis(tetrazole) borate sodium salt;
[0062] (2) Add 40mmol 1-ethyl-3-methyl-1H-imidazolium chloride salt and 44mmol bis(tetrazole) borate sodium salt to 100mL acetonitrile to obtain reaction solution II; reaction solution II was stirred at 25°C for 7 Days later, filter and collect the filtrate; after the acetonitrile solvent in the filtrate was removed by rotary evaporation, dissolve it with 50mL of dichloromethane, wash with water three times, then dry with anhydrous sodium sulfate, then carry out vacuum distillation, and finally vacuum ...
Embodiment 3
[0072] Synthesis of 1-ethylpyridine bis(tetrazole) borate
[0073] (1) Under the protection of argon, first add 106mmol of sodium borohydride to 70mL of anhydrous acetonitrile, and then add 212mmol of 1H-tetrazole in batches to obtain the reaction solution I; after the reaction solution I was refluxed and stirred at 85°C for 72h, Cool to room temperature, filter and collect solid matter, vacuum dry to obtain bis(tetrazole) borate sodium salt;
[0074] (2) Add 40mmol 1-ethylpyridinium bromide and 44mmol bis(tetrazole)borate sodium salt into 100mL of acetonitrile to obtain reaction solution II; reaction solution II was stirred and reacted at 25°C for 7 days, filtered and the filtrate was collected; After evaporating the acetonitrile solvent in the filtrate, first dissolve it with 50mL of dichloromethane, then wash it with water three times, then dry it with anhydrous sodium sulfate, then carry out vacuum distillation, and finally dry it in vacuum to obtain 8.809g 1-ethylpyridine b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com