Self-induction-based method for biomimetic titanification of immobilized protease
An immobilized protease self-induction technology, applied in the field of immobilized enzyme preparation, achieves the effects of mild conditions, simple immobilization process, and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] At room temperature, add 7.5mL 0.25mol / L Ti-BALDH solution to the immobilization reactor, and then add 22.5mL to the immobilization reactor to prepare the concentration of 18mg with pH 7.5 and 0.06mol / L phosphate buffer / mL free papain solution, so that the volume ratio of Ti-BALDH solution and papain solution is 1:3; gently stir for 5min, 5000rpm refrigerated centrifugation for 10min, and collect the precipitate. The precipitate was washed with distilled water for 3 times, the washing liquid was collected, the supernatant was combined, and then the precipitate was freeze-dried for 24 hours. The recovery rate of the obtained immobilized enzyme activity was 51.76%, and the encapsulation rate of papain was 78.67%.
Embodiment 2
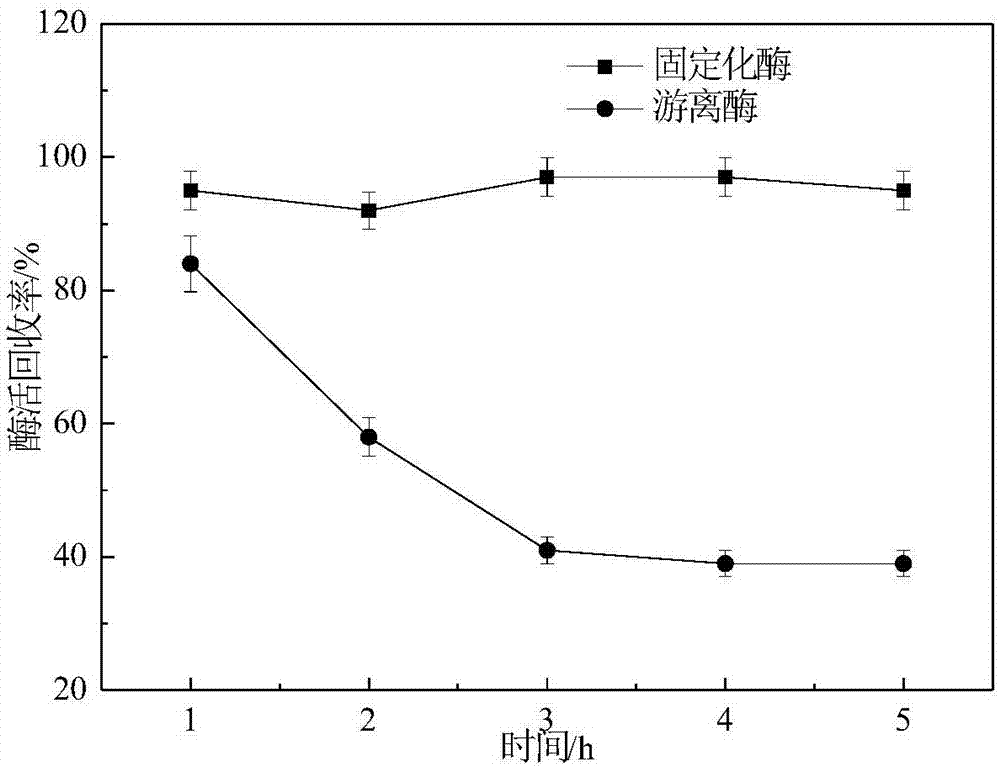
[0022] Take 20 mg of the immobilized papain and free protease obtained in Example 1 and incubate in a 55°C water bath, take a sample every 1h and add phosphate buffer at an optimal pH, then shake and react with casein in a 37°C water bath for 10 minutes, The residual enzyme activity was measured, and the enzyme activity of the sample without heat preservation treatment was taken as 100%, and the others were converted into relative enzyme activities to investigate the temperature stability of the immobilized papain. attached figure 1 is the relative enzyme activity of free and immobilized papain incubated at pH 7.5 with 55°C water bath for 5h. It can be seen from the figure that the free enzyme can maintain more than 80% of the relative enzyme activity after incubation in a 55°C water bath for 1 hour, but as the incubation time prolongs, the relative activity of free papain decreases rapidly, and only maintains the initial enzyme activity after 5 hours of incubation 39 percent...
Embodiment 3
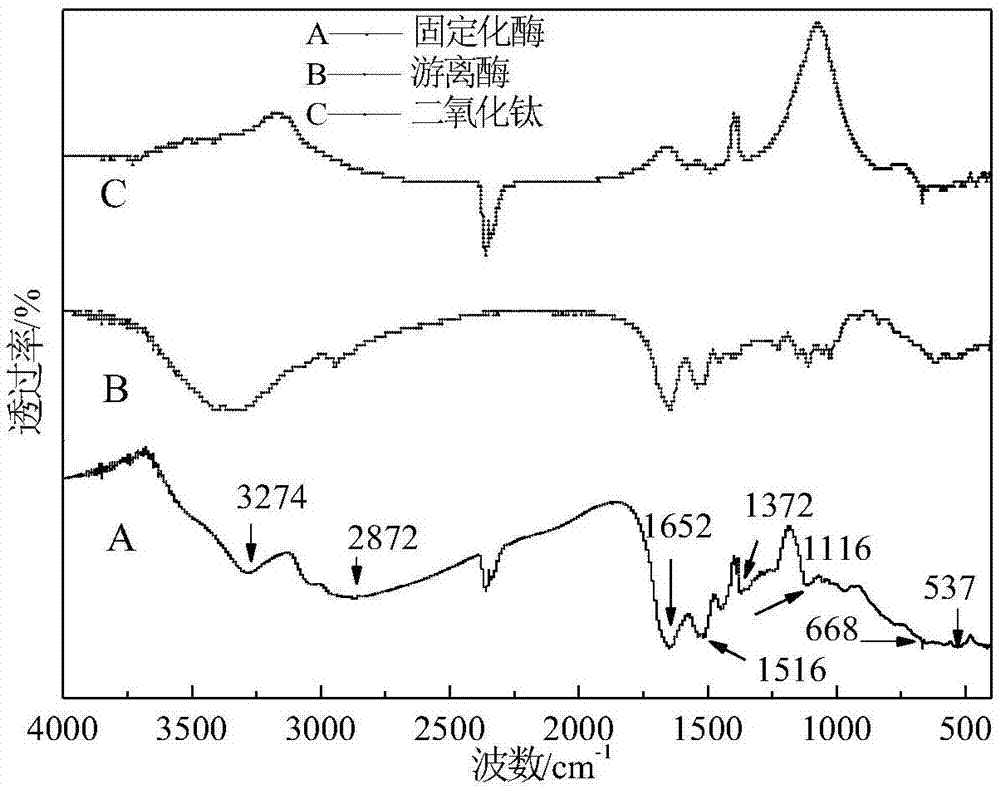
[0024] For the immobilized papain obtained in Example 1, after the sample is ground into fine pieces, potassium bromide is pressed into tablets, and its infrared spectrum is measured by a Fourier transform infrared spectrometer, and TiO is catalyzed with papain and ammonia water. 2 Infrared spectra for comparison, attached figure 2 shown. It can be seen from the figure that, compared with spectral line B, spectral line A has multiple absorption peaks of characteristic groups. Among them, 1372cm -1 It is the Ti-O-Ti antisymmetric stretching vibration absorption peak; 1116cm -1 It is the Ti-O-H stretching vibration absorption peak; 668cm -1 It is the Ti-O-Ti symmetrical stretching vibration absorption peak; 537cm -1 It is the bending vibration absorption peak of Ti-O-Ti, which further proves that TiO 2 generation. In addition, 1652 and 1516cm -1 The absorption peak at 2872cm is attributed to the vibrational absorption of the amide group (amide I band and II band) in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com