cTnI-cTnC-cTnT tripolymer protein and preparation method thereof, and cTnI detection kit
A ctni-ctnc-ctnt, trimer technology, applied in the field of time-resolved fluorescent quantitative detection kits, can solve the problems of protein insolubility and difficulty, and achieve the effects of stable labeled products, reliable results, and fast and easy detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
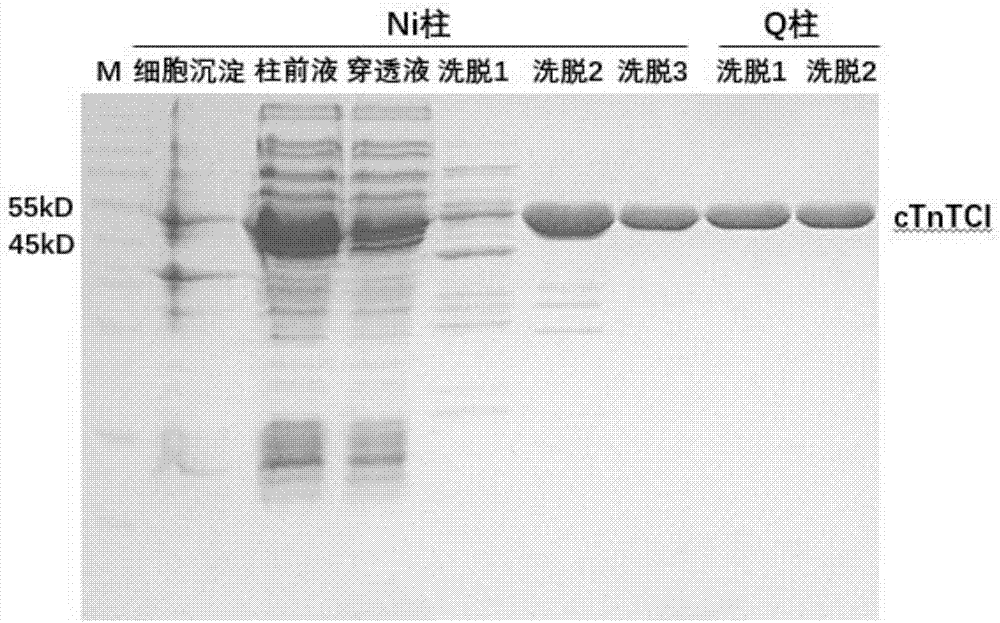
[0051] Embodiment 1: Cloning, expression and purification of cTnI fusion protein
[0052] ①According to the direction from the N-terminal to the C-terminal of the amino acid sequence, artificially synthesize the "cTnI-cTnC-cTnT" triplet gene sequence, as shown in SEQ ID NO.2.
[0053] ② The "cTnI-cTnC-cTnT" gene sequence was cloned into the multiple cloning insertion sites of BamH I and Xhol I of the pET28a plasmid expression vector to obtain the expression plasmid pET28a-cTnI.
[0054] ③ The cloned expression plasmid pET28a-cTnI was transformed into Escherichia coli BL21(DE3), plated and cultured overnight.
[0055] ④ The next morning, pick a single colony and inoculate it into LB medium, and culture it at 37°C until the optical absorption value (OD) of the bacterial solution reaches 1.0, and then add 1 μg / mL IPTG to induce expression. Bacteria were collected by centrifugation after incubation at 37°C for 4 hours.
[0056] ⑤ Add 100mL of 100mM Tris-HCl buffer (pH7.4) to the...
Embodiment 2
[0058] Embodiment 2: ELISA quantitative detection of cTnI active protein
[0059] ①The total protein content of the purified cTnI-cTnC-cTnT trimer protein was measured using the BCA protein content assay kit produced by Thermal Power Company, and the three batches of purified proteins were detected and error analysis was performed. The results are shown in Table 1.
[0060] ②Use the cTnI ELISA kit produced by CloneTech to detect the active protein content of cTnI. The above three batches of purified proteins were tested and analyzed for errors. The results are shown in Table 1.
[0061] Table 1. Determination of total protein content by BCA method and determination of cTnI active protein content by ELISA method
[0062]
Embodiment 3
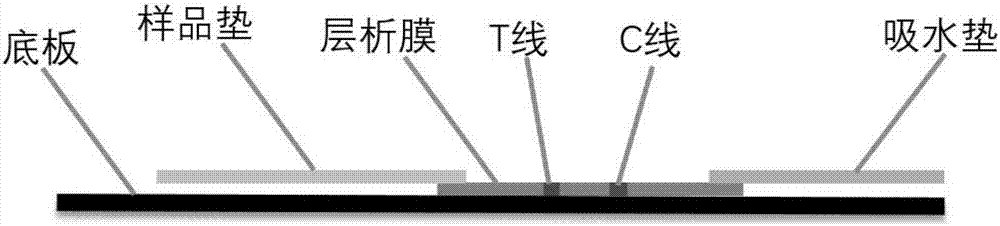
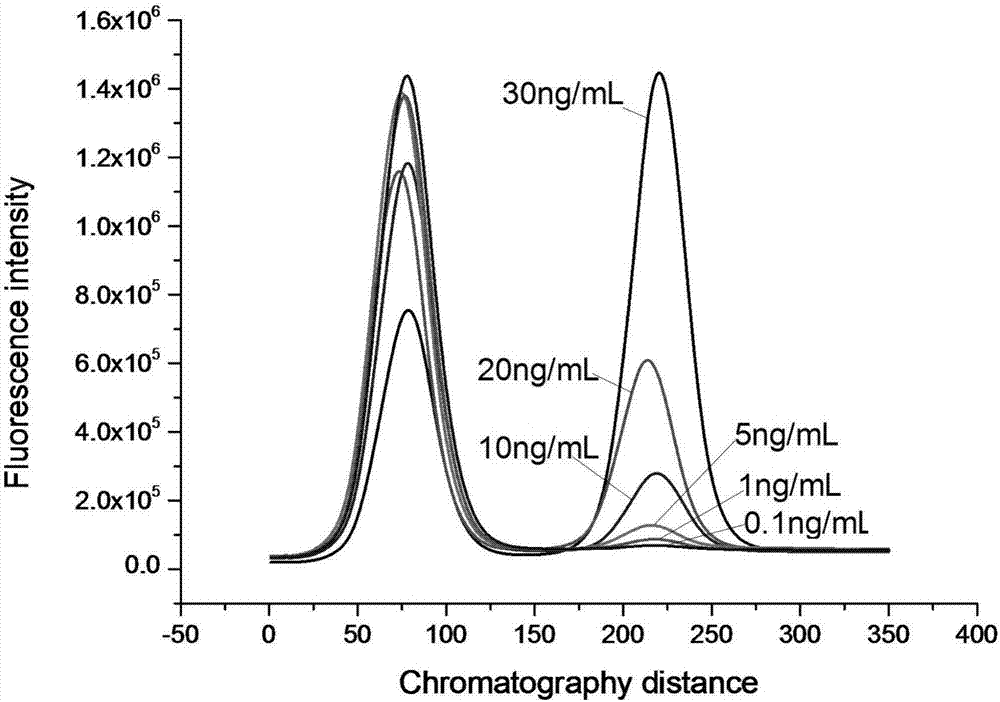
[0063] Embodiment 3: the preparation of immunochromatography kit
[0064] (1) Fluorescent microsphere-labeled antibody:
[0065] ① Take 0.5mL microspheres, centrifuge to get the precipitate, add MES buffer of pH 6.0 to wash twice.
[0066] ②Activation: 114 μL of 50 mM carbodiimide (EDAC) and 114 μL of 50 mM hydroxysulfosuccinimide (Sulfo-NHS) were added to the washed microspheres respectively, and shaken at room temperature for 1 h.
[0067] ③ Centrifuge the microspheres and wash twice with PB buffer.
[0068] ④ Add an appropriate amount of mouse-derived cTnI monoclonal antibody and shake at room temperature for 2 hours.
[0069] ⑤ Centrifuge the microspheres, add 50mM hydroxylamine buffer to quench the reaction, shake at room temperature for 5min; then centrifuge the microspheres, add 50mM hydroxylamine buffer to completely quench the reaction, shake at room temperature for 30min
[0070] ⑥ Centrifuge the microspheres, add blocking solution (0.5% casein, 10mM PB), shake at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com