Bacillus pumilus W3 CotA laccase mutant with improved soluble expression
A technology of Bacillus pumilus and mutants, applied in the field of bioengineering, can solve the problems of secondary pollution and poor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Construction of B. pumilus W3 laccase mutant
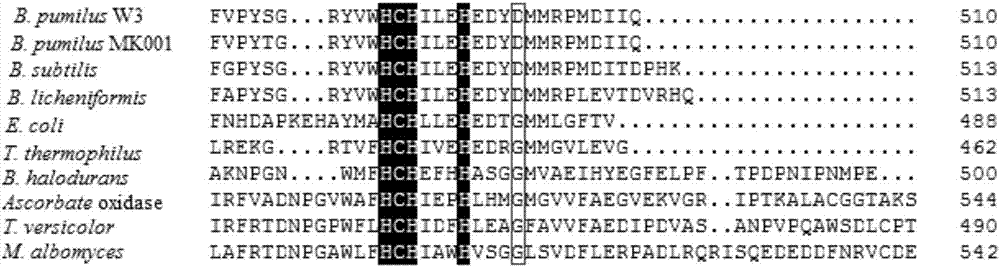
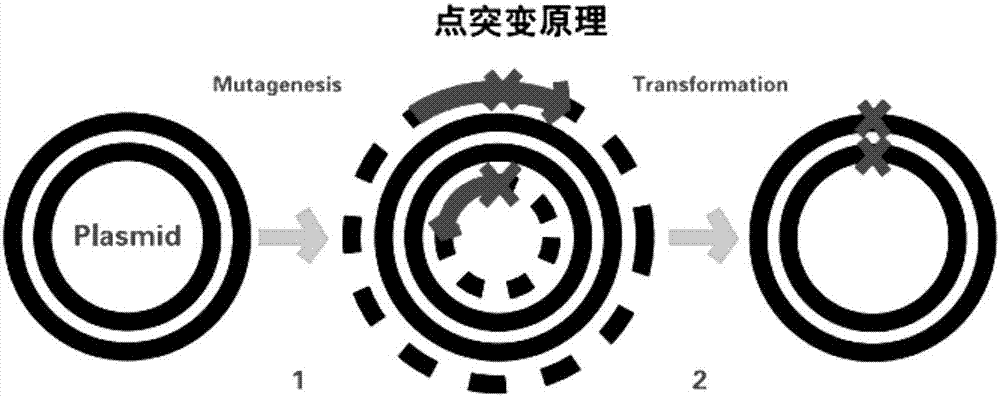
[0024] Principle of site-directed mutagenesis: The principle of point mutation uses primers to introduce mutation sites, and PCR SμperMix synthesizes mutant chains ( figure 2 ). Using the CotA laccase gene sequence of the recombinant laccase mutant WLF successfully constructed earlier as a template, primers were designed to mutate the 501st aspartic acid (Asp, D) in the laccase to glycine (Gly, D). The relevant forward primers and reverse primers designed are as follows:
[0025] Foreword F 5'-AACACGAGGATTAT GGC ATGATGCGGCC-3'
[0026] Back-quoted R 5'- CC ATAATCCTCGTGTTCTAATATGTGAC-3'
[0027]The underlined parts represent the codons corresponding to glycine at position 501 encoded by the mutant gene. The PCR amplification system is: plasmid DNA 3 μL, front primer (10 μM) 1 μL, back primer (10 μM) 1 μL, PCR SμperMix 25 μL, ddH 2 Make up to 50 μL with O, the PCR amplification conditions are denaturation ...
Embodiment 2
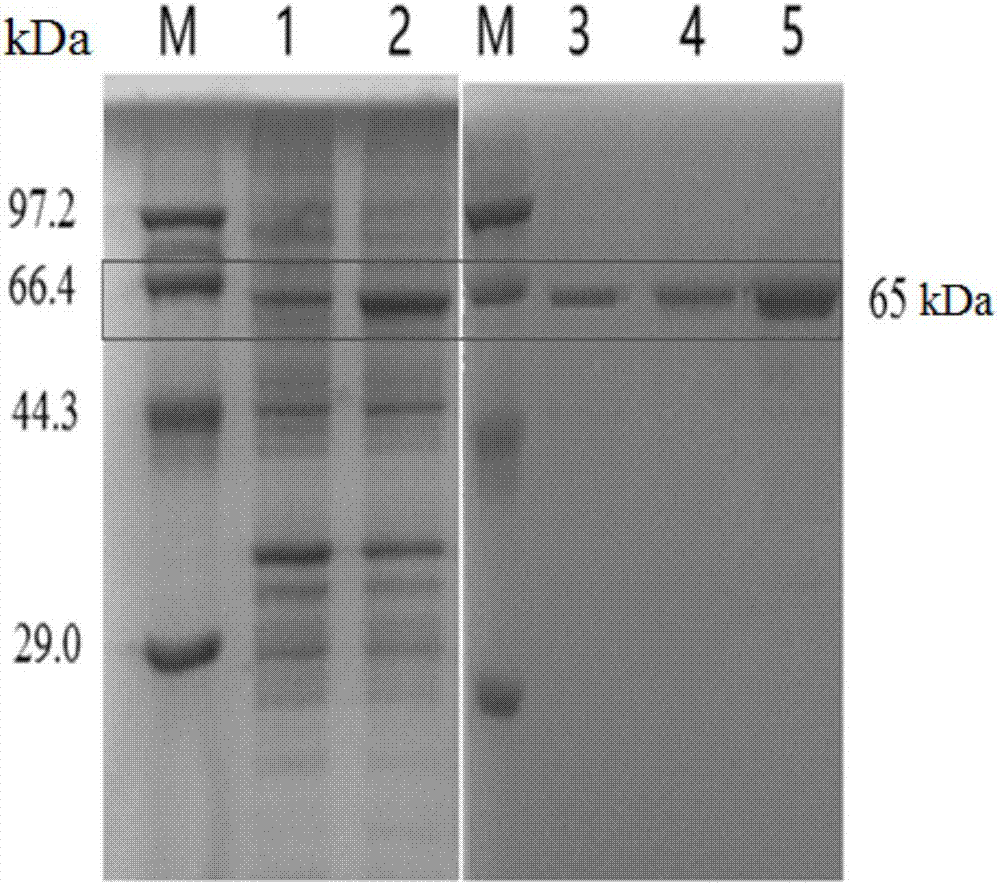
[0028] Example 2 Expression and purification of B.pumilus W3 laccase
[0029] Expression: Inoculate the wild-type recombinant laccase expression strain and previously constructed WLF and D501G / WLF recombinant expression strains from glycerol tubes into LB medium for activation, 37°C, 200rpm overnight (10h). The seeds were inserted into 50mL LB liquid fermentation medium (containing 100mg L -1 Ampicillin) 37 ℃ 200rpm shaker culture to OD 600 After reaching 0.5, the shaker temperature was adjusted to 15°C for static culture for 30min, then the final concentration of 0.4mM IPTG and 0.25mM CuSO4 were added to induce, and cultured at 15°C for 24h at 200rpm, the fermentation broth was centrifuged at 4°C at 8000rpm for 10min to remove the supernatant and collect bacteria. Resuspend the collected bacteria with phosphate buffer, and after resuspension, use an ultrasonic cell disruptor to crush the bacteria to release intracellular proteins. The supernatant was heated in a water bath...
Embodiment 3
[0031] Example 3 Enzyme activity determination and expression analysis of B.pumilus W3 laccase
[0032] (1) Definition of enzyme activity unit: When using the ABTS method to determine the enzyme activity of laccase, define the amount of enzyme required to catalyze the conversion of 1 μmol of substrate into product per minute as an activity unit.
[0033] (2) Enzyme activity assay steps: 1 Preheating: Take 2.4mL of pH4.0 citrate buffer solution in a test tube, add 0.5mL ABTS solution (the final concentration of ABTS is 0.5mM) into the test tube and place it in a water bath at 50°C Preheat for 5 minutes; 2 reactions: Add 0.1mL of diluted sample enzyme solution and shake evenly. 3 Measurement: Use a spectrophotometer to measure the kinetics of the uniformly shaken sample, measure the change in OD value per minute within 30s at a wavelength of 420nm (the measurement reaction shows a straight line) and calculate the enzyme activity.
[0034] (3) Determination of kinetic parameters...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decolorization rate | aaaaa | aaaaa |
| decolorization rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com