Preparation method of propiolic acid compounds
A compound, propynoic acid technology, applied in the field of preparation of propynoic acid compounds, can solve the problems of large ligands, difficult to handle, difficult to synthesize, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
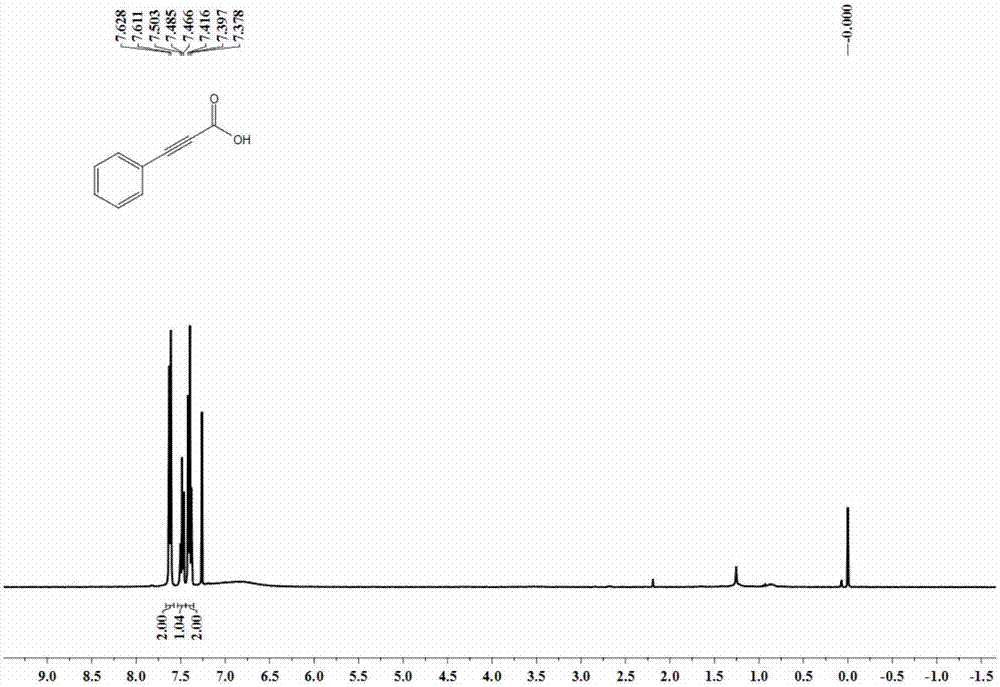
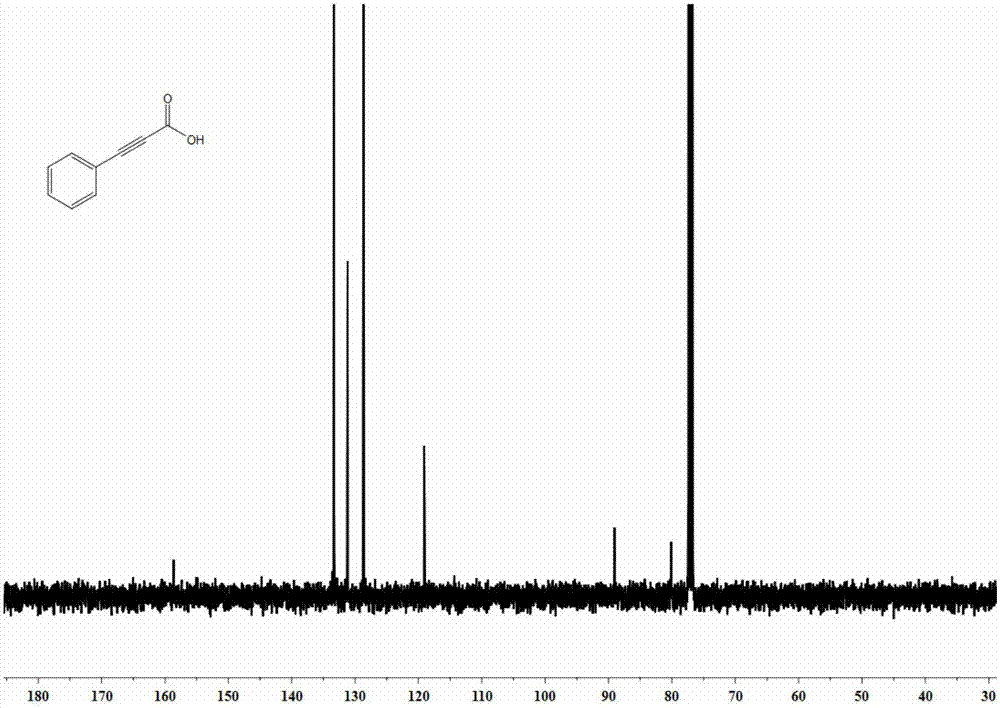
[0037] The synthesis of embodiment 1 phenylpropiolic acid
[0038]
[0039] Weigh cuprous chloride (9.9mg, 0.1mmol), potassium carbonate (552mg, 4mmol), and tetrabutylammonium acetate (451.5mg, 1.5mmol), and add them to a 25mL reaction kettle in turn, and replace the nitrogen with vacuum three times. Add refined acetonitrile (4.0mL) and phenylacetylene (102mg, 1mmol) under nitrogen protection, fill with CO 2 (0.1 MPa). Close the reaction kettle, place it in an oil bath at 25°C, and react for 20 hours. After the reaction, open the valve on the reaction kettle to slowly release the remaining gas, then transfer the reaction solution in the reaction kettle to a one-mouth bottle for concentration, and use 5mL deionized Dilute with water, then extract with n-hexane, add 1M hydrochloric acid to acidify the aqueous layer to pH = 1 at low temperature, then extract with ether, collect the organic phase, wash with saturated brine, dry with anhydrous sodium sulfate, filter, and remove t...
Embodiment 2
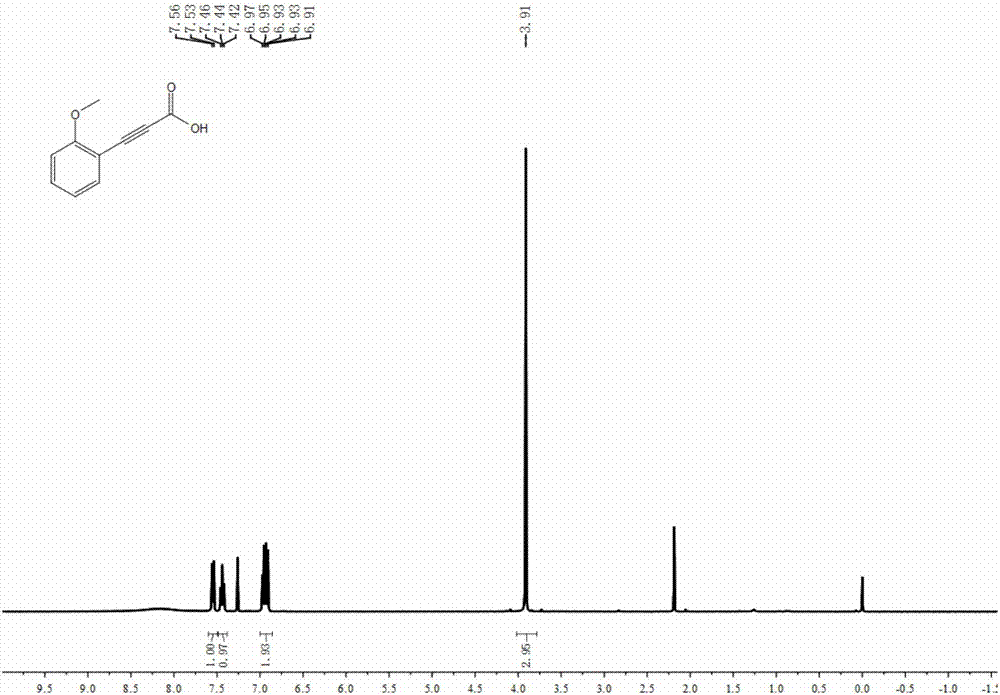
[0040] The synthesis of embodiment 2 2-methoxyphenylpropynoic acid
[0041]
[0042] Weigh cuprous iodide (9.5mg, 0.05mmol), sodium carbonate (530mg, 5mmol), tetrabutylammonium nitrite (288mg, 1mmol), 2-methoxyphenylacetylene (132mg, 1mmol), add to In the 25mL reaction kettle, the nitrogen was evacuated three times, and the refined THF (4.0mL) was added under the protection of nitrogen, and the CO was filled. 2 (0.1 MPa). Close the reaction kettle and place it in an oil bath at 25°C to react for 16 hours. After the reaction, open the valve on the reaction kettle to slowly release the remaining gas, then transfer the reaction liquid in the reaction kettle to a one-mouth bottle for concentration, and use 5mL deionized water to Dilute, then extract with n-hexane, add 1M hydrochloric acid to the aqueous layer and acidify to pH = 1 at low temperature, then extract with ether, collect the organic phase, wash with saturated brine, dry over anhydrous sodium sulfate, filter, remove...
Embodiment 3
[0043] The synthesis of embodiment 3 4-chlorophenylpropynoic acid
[0044]
[0045] Weigh copper acetate (10mg, 0.05mmol), potassium carbonate (552mg, 4mmol), n-tetrabutylammonium bromide (644.6mg, 2mmol), sodium acetate (272.2mg, 2mmol) 4-chlorophenylacetylene (136.5mg, 1mmol) ), were added to a 25mL reaction kettle in turn, and the nitrogen was replaced by vacuum three times. Under the protection of nitrogen, refined dichloromethane (5.0mL) was added, filled with CO 2 (0.1MPa). Close the reaction kettle and place it in an oil bath at 25°C for 18 hours of reaction. After the reaction is over, open the valve on the reaction kettle to slowly release the remaining gas. Dilute, then extract with n-hexane, add 1M hydrochloric acid to the aqueous layer to acidify to pH = 1 at low temperature, then extract with ether, collect the organic phase, wash with saturated brine, dry with anhydrous sodium sulfate, filter, remove the solvent in vacuo, 4-Chlorophenylpropynoic acid was obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com