Preparation method of pidotimod
A technology of pidotimod and thiazolidine, applied in the field of drug synthesis, can solve the problems of irritation to eyes, skin and respiratory tract, low product yield and purity, prominent environmental problems, etc., and achieves good practicability, fewer synthesis steps, and reduced The effect of large-scale production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
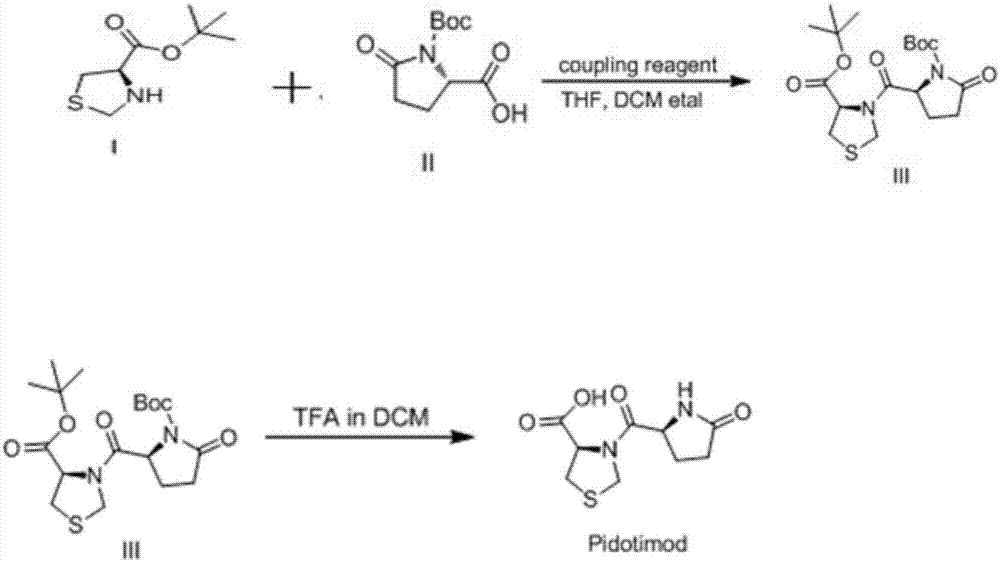
[0041] (1) Condensation reaction
[0042]In a 250mL reaction flask, dissolve 9.5g (0.05mol) of L-thiazolidine-4-carboxylate tert-butyl ester and 12g (0.050mol) of L-(tert-butoxycarbonyl)-pyroglutamic acid in 150ml of dichloromethane , stirred and cooled to 2°C, added dropwise 14ml (0.1mol) of triethylamine, followed by adding 10g (0.055mol) of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 8 g (0.055 mol) of 1-hydroxybenzotriazole monohydrate, naturally warmed to room temperature and stirred for 12 hours, 300 ml of dichloromethane diluted the reaction solution, and then washed with 1N hydrochloric acid, saturated sodium bicarbonate, and saturated brine respectively, The organic phase was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain 19 g of yellow oil, (4R)-3-[[(2S)-5-oxo-2(tert-butoxycarbonyl)-2- Pyrrolidinyl]carbonyl]-4-thiazolidinecarboxylic acid tert-butyl ester, the yield is 9...
Embodiment 2
[0046] (1) Condensation reaction
[0047] In a 250mL reaction flask, dissolve (190g (1mol) of tert-butyl L-thiazolidine-4-carboxylate) and 240g (1mol) of L-(tert-butoxycarbonyl)-pyroglutamic acid in 3L of dichloromethane , stirred and cooled to 2°C, added dropwise 280ml (2mol) of triethylamine, followed by adding 200g (1.1mol) of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 1 -Hydroxybenzotriazole monohydrate 160g (1.1mol), naturally warming to room temperature and stirring for 12h, using 6L of dichloromethane to dilute the reaction solution, and then washing with 1N hydrochloric acid, saturated sodium bicarbonate, saturated brine, organic Dry over anhydrous sodium sulfate, filter, and concentrate the filtrate to dryness under reduced pressure to obtain 400 g of yellow oil, (4R)-3-[[(2S)-5-oxo-2(tert-butoxycarbonyl)-2-pyrrole Alkyl]carbonyl]-4-thiazolidinecarboxylic acid tert-butyl ester, the yield is 94.7%.
[0048] (2) Hydrolysis reaction
[0049] Disso...
Embodiment 3
[0051] (1) Condensation reaction
[0052] Take 190 g of (L-thiazolidine-4-carboxylate tert-butyl ester, 240 g of L-(tert-butoxycarbonyl)-pyroglutamic acid) and dissolve them in 3L of tetrahydrofuran solvent, stir and cool to 4°C, add three Ethylamine 280ml, then add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 200g and 1-hydroxybenzotriazole monohydrate 160g, naturally warming up to room temperature, reaction 10 The reaction solution was washed with 1N hydrochloric acid, saturated sodium bicarbonate solution, and saturated saline solution, the organic phase was dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness under reduced pressure to obtain yellow oil (4R)-3-[[ 386 g of tert-butyl (2S)-5-oxo-2(tert-butoxycarbonyl)-2-pyrrolidinyl]carbonyl]-4-thiazolidinecarboxylate, the yield was 91.4%.
[0053] (2) Hydrolysis reaction
[0054] Take (4R)-3-[[(2S)-5-oxo-2(tert-butoxycarbonyl)-2-pyrrolidinyl]carbonyl]-4-thiazolidinecarb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com