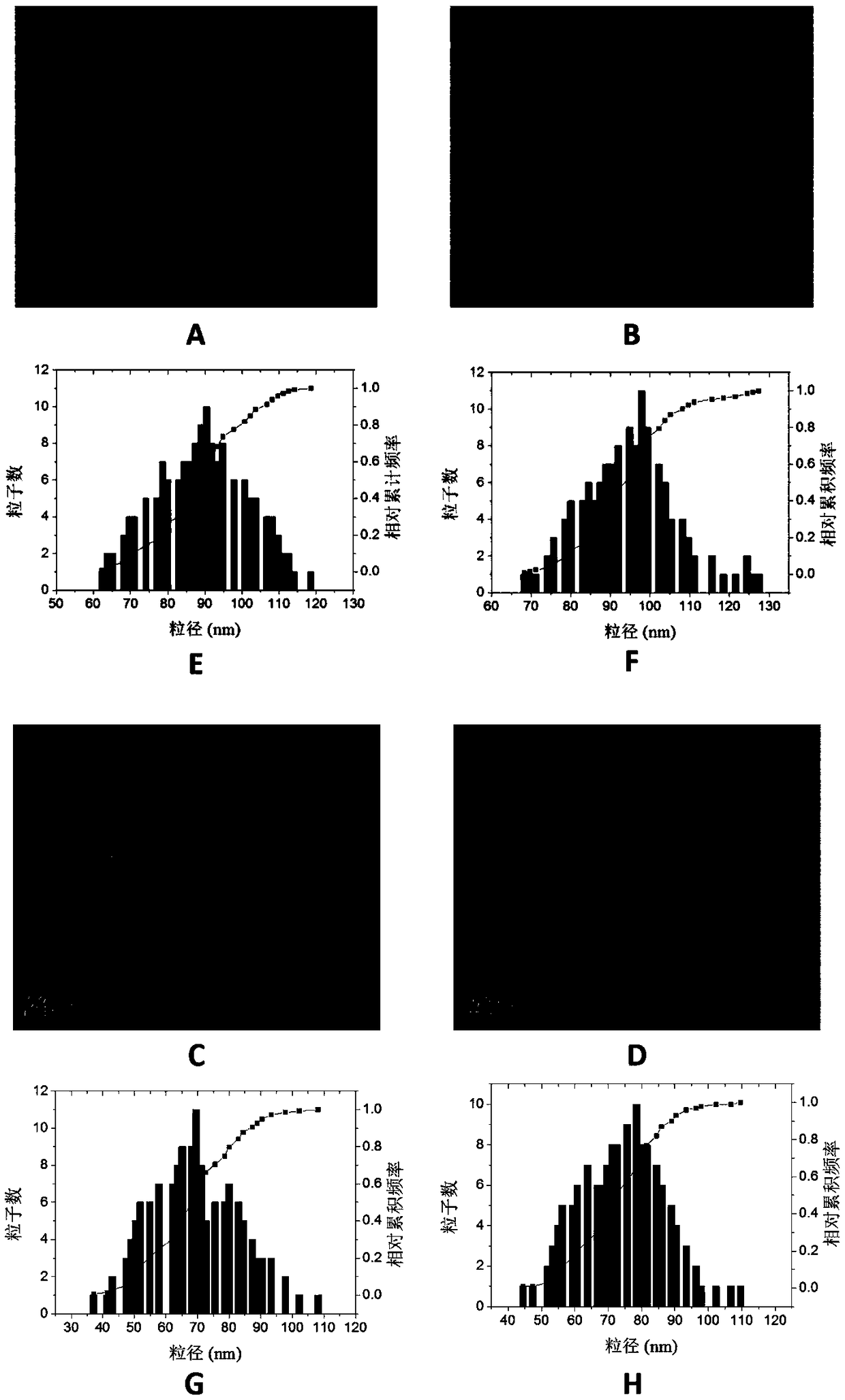
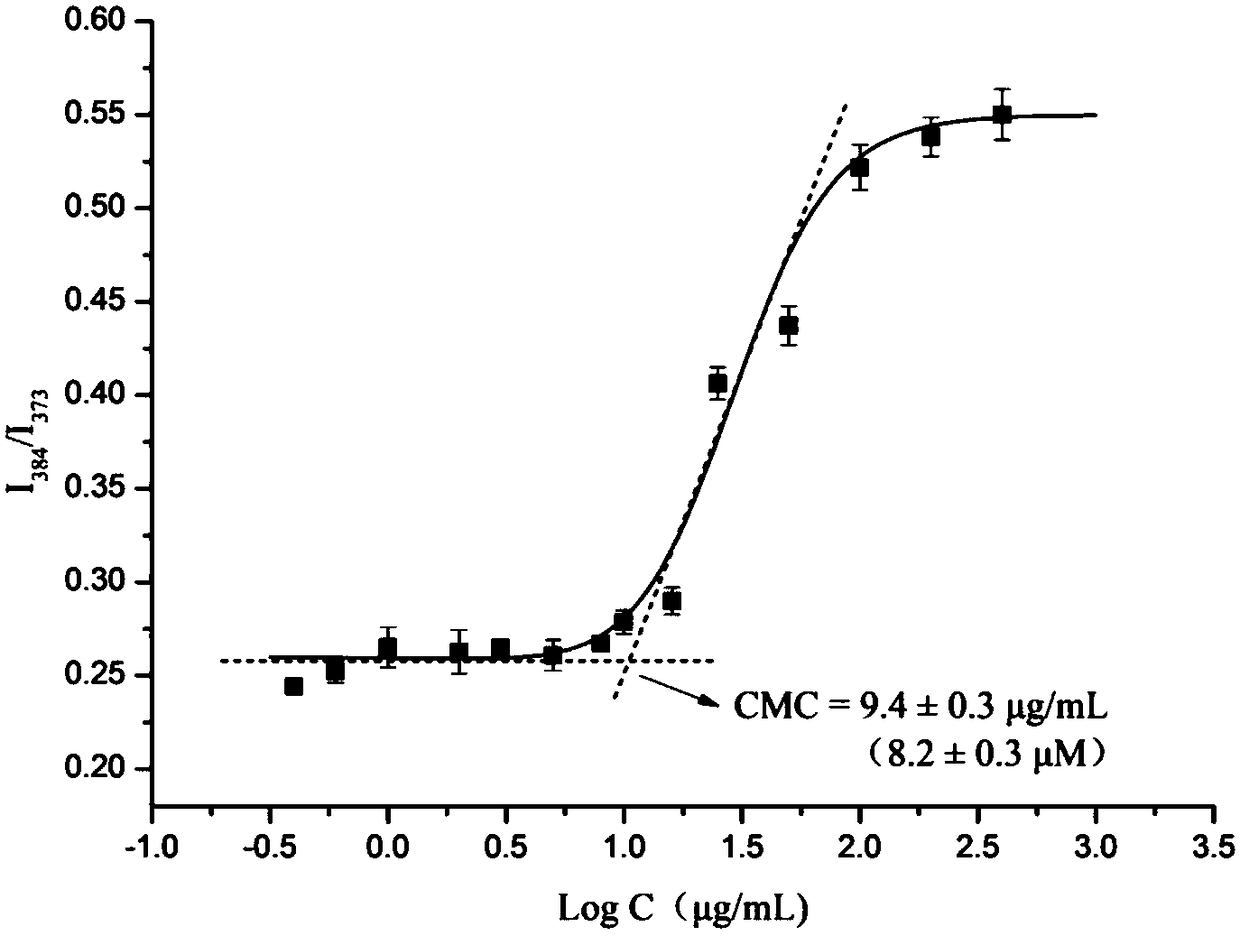
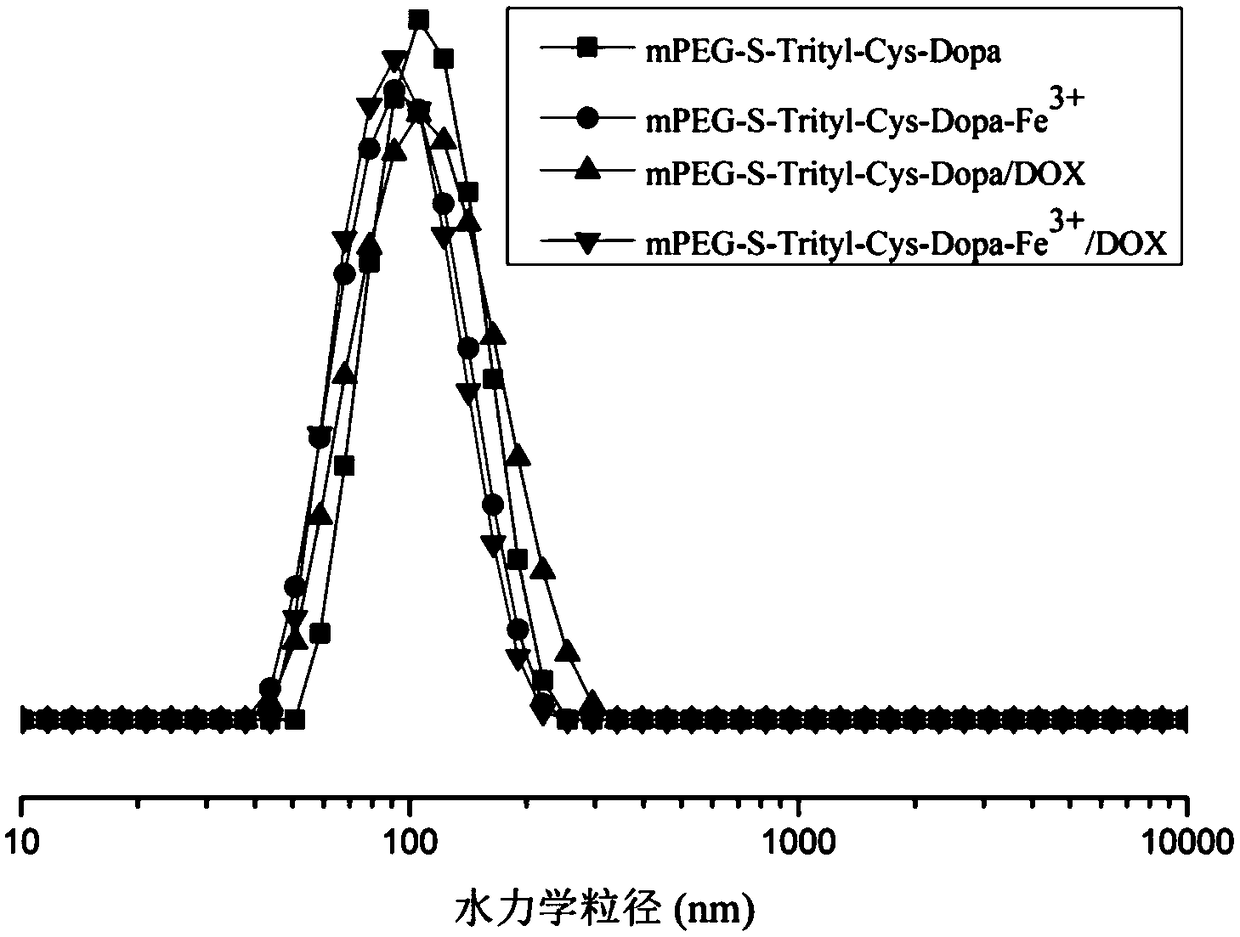
A kind of multifunctional imaging cross-linked stable nanometer drug-loaded micelles and its preparation method
A drug and carrier technology, which is applied in the field of preparation of acid-sensitive nanomicelles, can solve the problems of sudden release of drugs, inability to release drugs completely, and reduced cycle time, so as to overcome poor stability and targeting, instability and High toxicity and side effects, improved stability and dilution resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Amphiphilic drug carrier mPEG-S-Trityl-Cys-Dopa, represented by formula I:
[0066]
[0067] where n=9;
[0068] The mPEG-S-Trityl-Cys-Dopa is the abbreviation of polyethylene glycol monomethyl ether-S-trityl-L-cysteine-dopamine.
[0069] n can also be 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 or 25.
[0070] In the present invention, dopamine is introduced into the carrier in the form of amidation, which is equivalent to the introduction of catechol functional groups, and Fe is added under the condition of pH 7.4 3+ Cross-linked with catechol to prepare carrier cross-linked micelles mPEG-S-Trityl-Cys-Dopa-Fe 3 + , the carrier cross-linked drug-encapsulated micelles mPEG-S-Trityl-Cys-Dopa-Fe were prepared by encapsulating the anticancer drug doxorubicin (DOX) by dialysis 3+ / DOX. This prodrug micelle itself is stable in the blood circulation, and effectively releases drugs under the acidic microenvironmental conditions in tumor cells to achie...
Embodiment 2
[0076]The preparation method of amphiphilic drug carrier mPEG-S-Trityl-Cys-Dopa is characterized in that it comprises the following steps:
[0077] (1) 1.1 g of S-trityl-L-cysteine (II), di-tert-butyl dicarbonate (Boc 2 O) 1.0g and 19mL of 2N aqueous sodium hydroxide solution, react at room temperature for 24h, after the reaction is completed, lower to room temperature, slowly add 1mol / L HCl to adjust the pH to = 2, extract the aqueous layer with dichloromethane, combine the organic layers with distilled water Wash with water until the pH is 7, add anhydrous sodium sulfate to the organic layer to dry and remove water, collect the filtrate by suction filtration, and rotary evaporate the filtrate to remove the extractant to obtain white foamy solid amino-protected-S-trityl-L-cysteine (N-Boc-S-Trityl-Cys) (III);
[0078] The nuclear magnetic and mass spectral data of this compound (III) are as follows: 1 H NMR (CDCl 3 ,400MHz):δ7.21-7.41(m,15H,S-trityl-H),4.91(d,1H,CONH),3.9...
Embodiment 3
[0089] The preparation method of amphiphilic drug carrier mPEG-S-Trityl-Cys-Dopa is characterized in that it comprises the following steps:
[0090] (1) 1.1 g of S-trityl-L-cysteine (II), di-tert-butyl dicarbonate (Boc 2 (0) 1.0 g and 19 mL of 2N aqueous sodium hydroxide solution were reacted at room temperature for 24 hours. After the reaction was completed, the temperature was lowered to room temperature, and 1 mol / L HCl was slowly added dropwise to adjust the pH to = 1. The aqueous layer was extracted with dichloromethane, and the combined organic layers were washed with distilled water. When the pH was 7, the organic layer was dried by adding anhydrous sodium sulfate to remove water, the filtrate was collected by suction filtration, the filtrate was rotary evaporated to remove the extractant, and a white foamy solid amino-protected-S-trityl-L-cysteine ( N-Boc-S-Trityl-Cys) (III);
[0091] (2) Formula (III) compound 1.3g, catalyst 1-hydroxybenzotriazole 0.8g and dehydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com