A sugar-resistant and acid-resistant β-glucosidase and its production method
A glucosidase and sugar-tolerant technology, applied in the biological field, can solve the problems of limited large-scale application and high price, and achieve the effects of large-scale production and use, good thermal stability and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
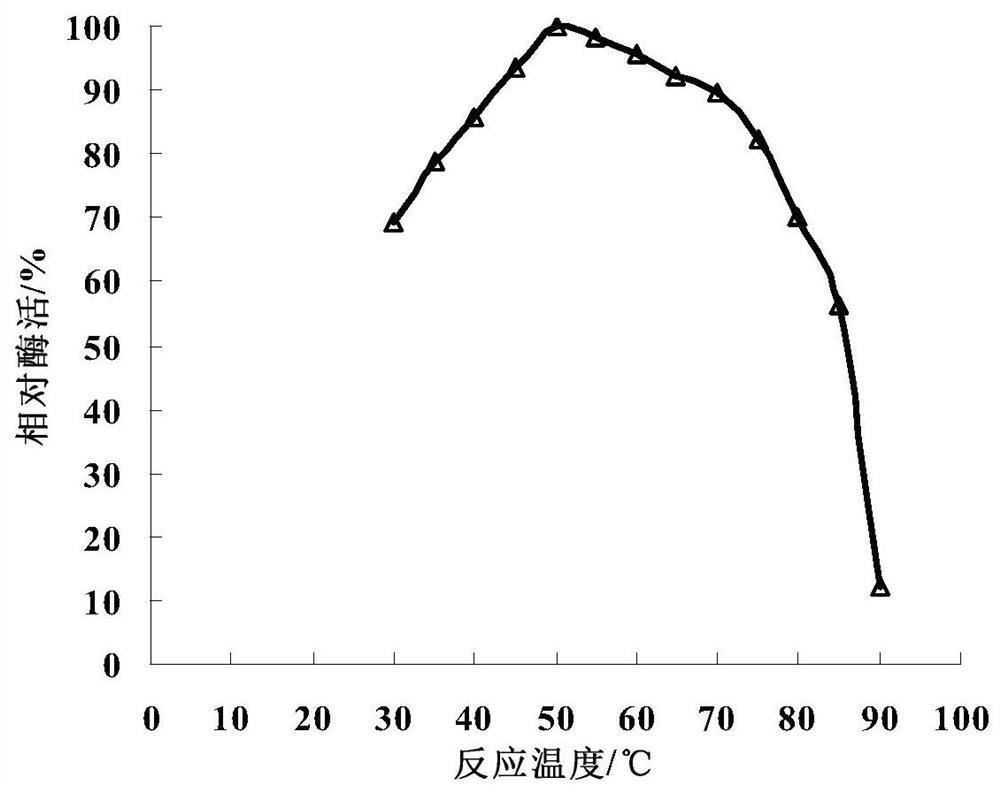
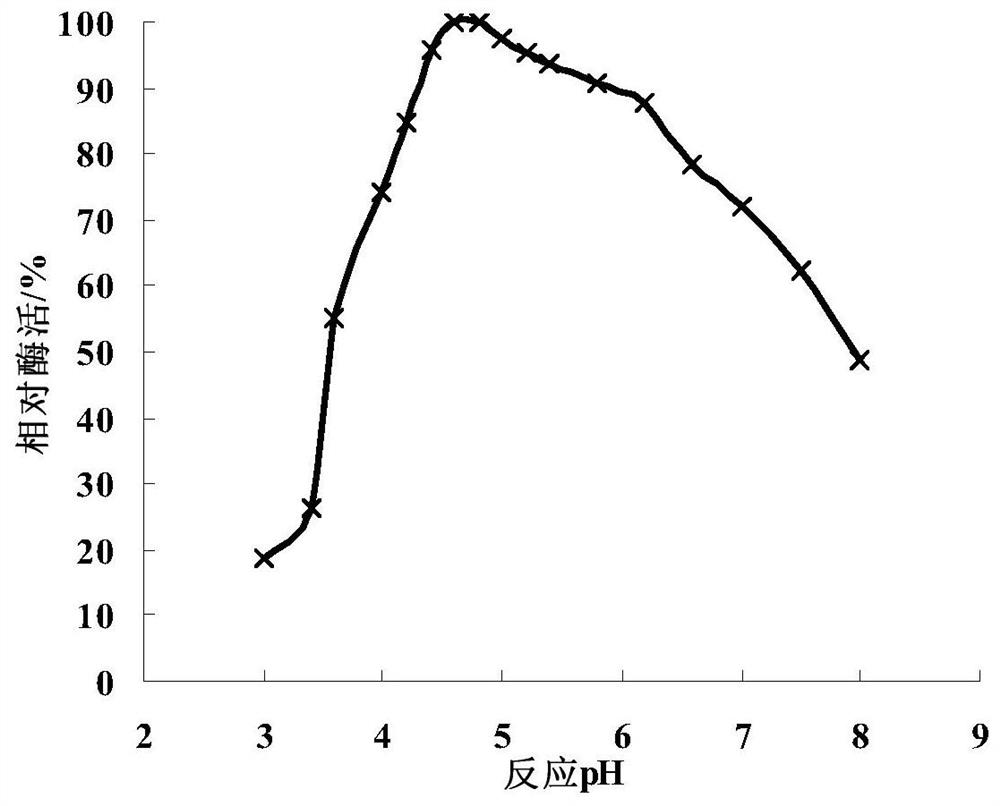
Image
Examples
Embodiment 1
[0021] Example 1Co 60 Mutagenesis and strain screening
[0022] Preparation of spore suspension: Take sterile physiological saline to wash the spores on the fresh slant of the original strain, transfer them into a triangular flask filled with glass beads, vibrate and disperse them on a shaker for 60 minutes to make a bacterial suspension, and carry out gradient Dilute, count on a hemocytometer and adjust the spore concentration to 10 6 A / mL or so.
[0023] co 60 Radiation mutagenesis: place a test tube containing an appropriate amount of spore suspension on a radiation plane with a certain dose rate, and use Co 60 Radiation mutagenesis was carried out with irradiation doses of 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0 kGy respectively. After treatment, gradient dilution was carried out, spread on a PDA medium plate, and cultivated at 30°C.
[0024] Determination of mutagen dose: make 10-fold serial dilutions of the mutagenized and non-mutated bacterial suspensions, draw 1mL o...
Embodiment 2
[0036] Example 2 Beta-glucosidase enzyme activity assay
[0037] Enzyme activity definition: the amount of enzyme required to release 1 μmol p-NP per minute is an enzyme activity unit U.
[0038] Determination method: centrifuge the fermentation broth at 3000r / min for 10min, and get the supernatant as the crude enzyme solution. Accurately absorb 0.1mL of the crude enzyme solution, add 0.9mL of disodium hydrogen phosphate-citric acid buffer solution with pH4.8, and place in a constant temperature water bath at 50°C Preheat for 5 minutes, add 1 mL of preheated 5 mmol / L p-NPG, react with a stopwatch at 50°C for 10 minutes, and immediately add 1 mL of 1 mol / L sodium carbonate solution to terminate the reaction. Absorbance was measured at 420 nm.
Embodiment 3
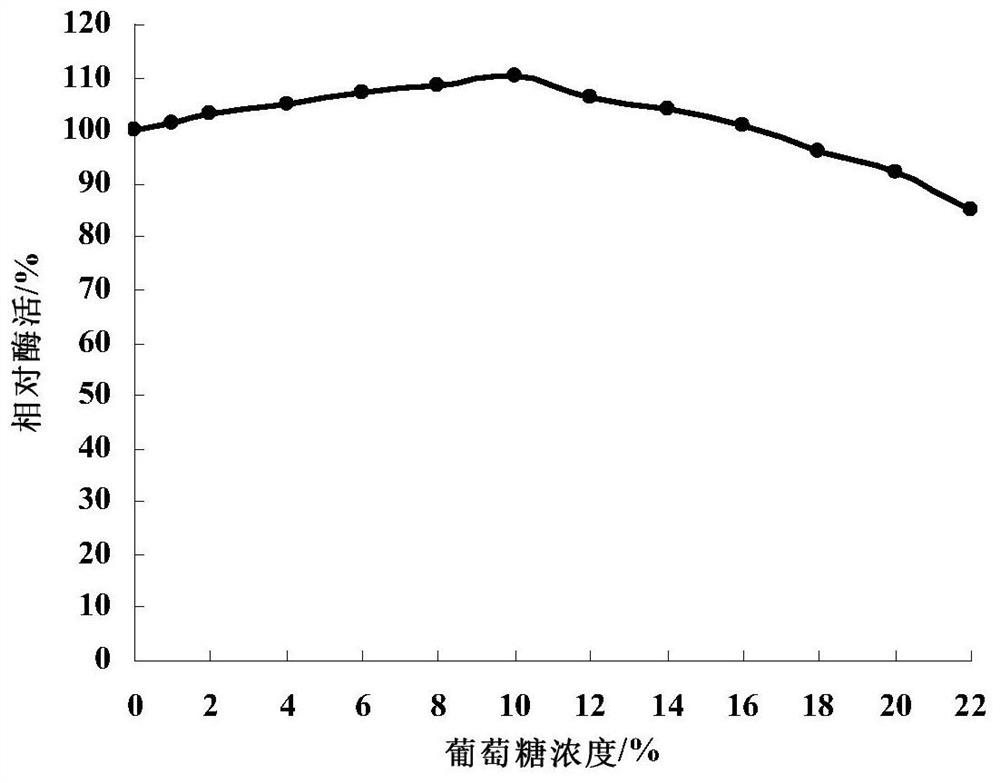
[0039] The glucose tolerance of embodiment 3β-glucosidase
[0040] Taking the supernatant of AN-4 fermented liquid obtained in Example 1 as a sample, add 0-18% glucose respectively in the reaction system, determine the activity of β-glucosidase according to the method described in Example 2, and obtain the relative enzyme activity change curve like figure 1 , when the glucose concentration is lower than 13%, it can enhance the enzyme activity, the highest enhancement is 10%, and at the glucose concentration of 14%, the enzyme activity still retains about 95%, indicating that the β-glucosidase produced by AN-1 has Higher glucose tolerance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com