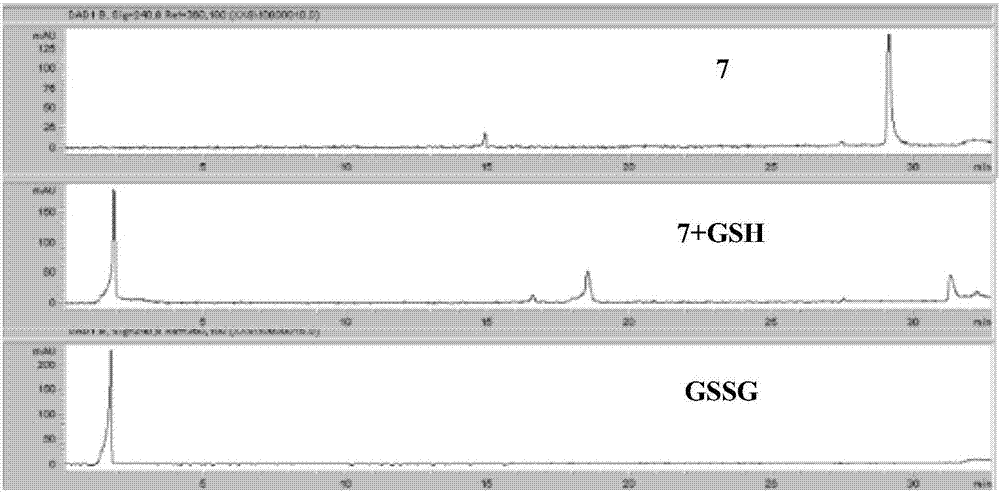
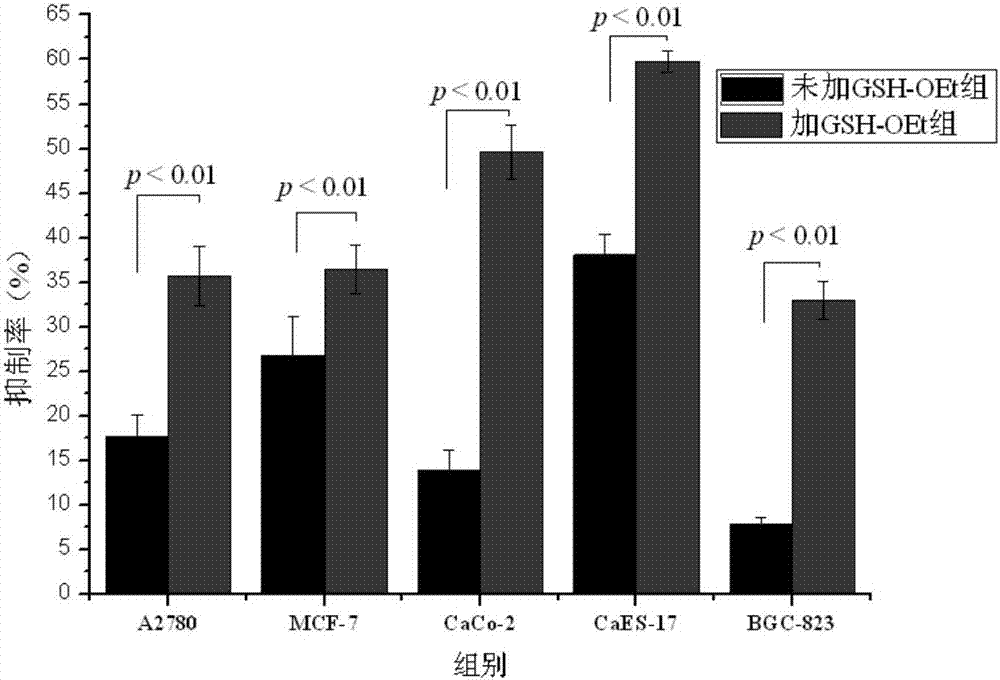
Redox-responsive cyclodextrin-modified 1-methyl-beta-carboline-3-carboxylic acid conjugate, and preparation method and application thereof
A technology of cyclodextrin and conjugates, which is applied in redox-responsive cyclodextrin-modified 1-methyl-β-carboline-3-carboxylic acid conjugates and its preparation and application fields, which can solve Issues such as responsive response and reduced toxicity of doxorubicin have not been disclosed, and the effects of improving solubility and bioavailability, excellent anti-tumor activity, and reducing toxicity have been achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
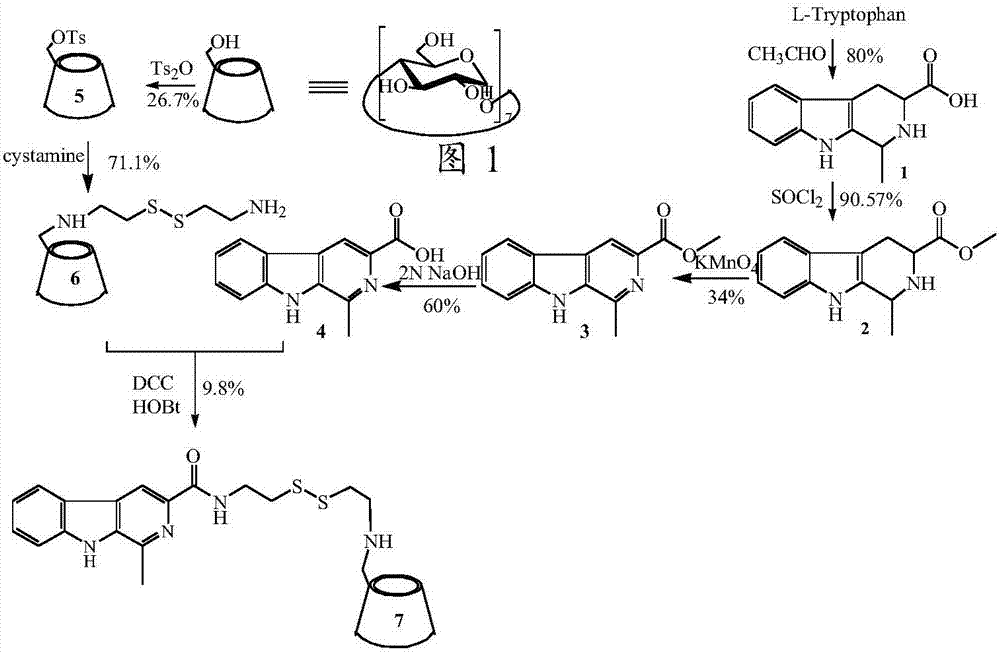
[0032] Example 1 Preparation of (3S)-1,2,3,4-tetrahydromethyl-β-carboline-3-carboxylic acid (1)
[0033] Add 0.2ml of concentrated sulfuric acid dropwise to 80ml of water, add L-tryptophan (2.0g, 9.79mmol) into the aqueous solution, dissolve it by ultrasonic, add 2ml of 40% acetaldehyde solution, and stir at room temperature for 6h. When the reaction was terminated, ammonia water was added to adjust the pH to 6-7, and the mixture was left to stand overnight in a refrigerator at 4°C. Filtration under reduced pressure gave a white solid (1.8 g, 80%). ESI / MS(m / z):231[M+H] + .
Embodiment 2
[0034] Example 2 Preparation of (3S)-1,2,3,4-tetrahydromethyl-β-carboline-3-carboxylic acid methyl ester (2)
[0035] Add 75ml of methanol to a 250ml eggplant bottle, add thionyl chloride (6.6ml, 60.06mmol) dropwise under ice bath, stir for 30min under ice bath, then slowly add (3S)-1,2,3,4-tetrahydro Methyl-β-carboline-3-carboxylic acid (5 g, 21.64 mmol) was reacted for 9-10 h, the reaction solvent was drained, and 20 ml of diethyl ether was added to grind and wash 3 times to obtain a white solid. The obtained white solid was dissolved in 100ml of ethyl acetate, added to saturated sodium bicarbonate solution and stirred at room temperature for 30min and then extracted, the ethyl acetate layer was dried with anhydrous sodium sulfate for 30min, and the ethyl acetate was spun off to obtain a light yellow oil (4.8 g, 90.57%). ESI / MS(m / z):245[M+H] + .
Embodiment 3
[0036] Example 3 Preparation of 1-methyl-β-carboline-3-carboxylic acid methyl ester (3)
[0037] Add (3S)-1,2,3,4-tetrahydromethyl-β-carboline-3-carboxylic acid methyl ester (4.8g, 19.59mmol) into a 250ml eggplant bottle, add acetone under ice-cooling to dissolve it , Potassium permanganate (6.2g, 39.24mmol) was added several times in small amounts, the solvent was removed after reacting at room temperature for 6h, and 50ml of methanol was added to redissolve. Column chromatography separation and purification (dichloromethane:methanol=60:1, R f =0.3) gave a white solid (1.6 g, 34%). ESI / MS(m / z):241[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com