Three-dimensional N-heterocyclic carbene metal coordination polymer, preparation method and application thereof
A nitrogen-heterocyclic carbene and metal coordination technology, which is applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, catalytic reactions, etc. Stability and other issues, to achieve the effect of good stability, high catalytic activity, efficient recovery and reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
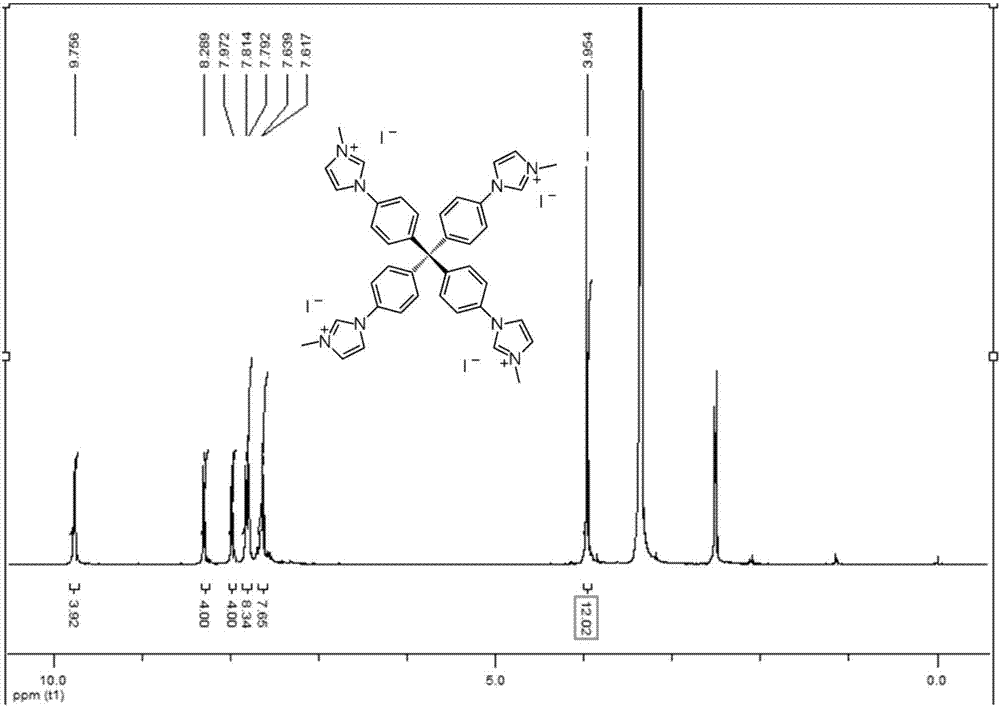
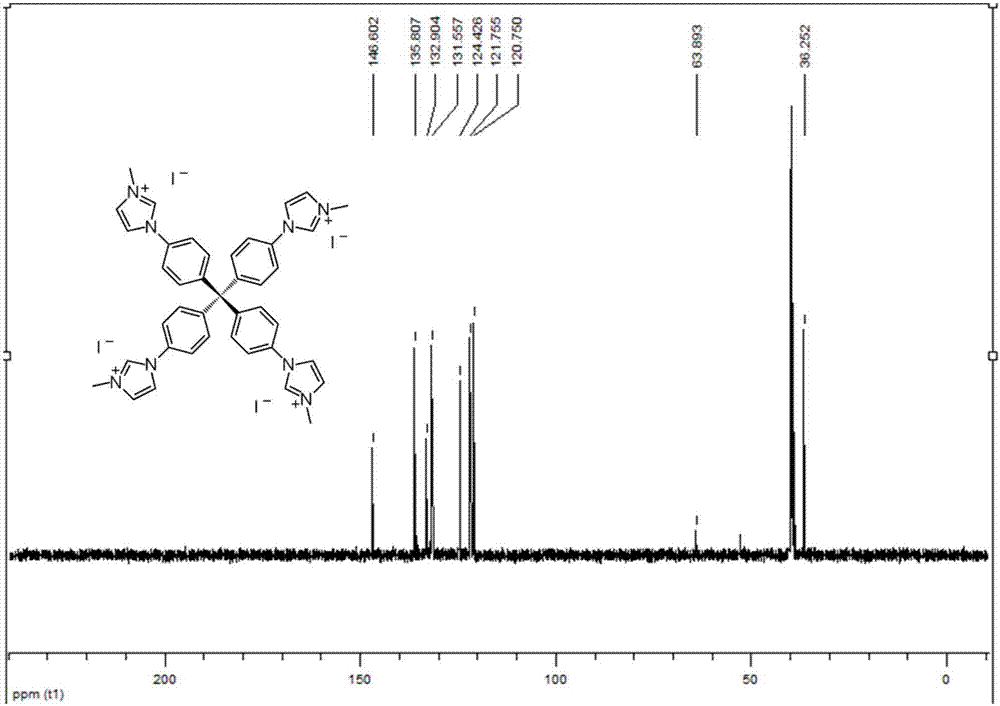
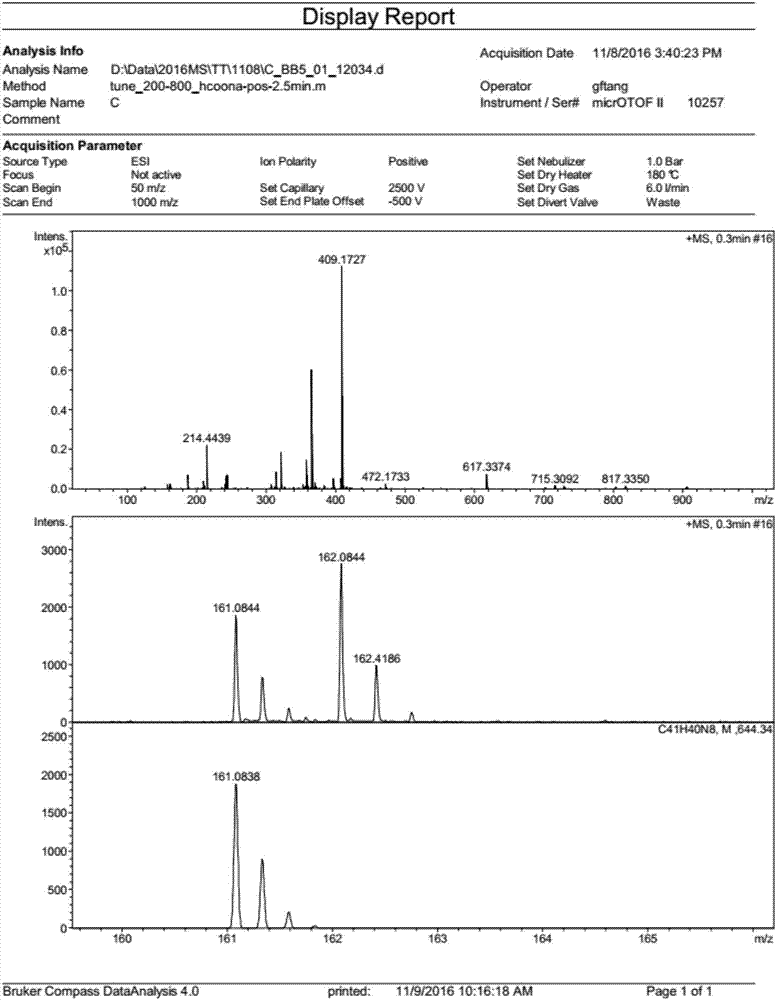
[0062] Example 1, the preparation of tetraphenylmethane tetraimidazolium salt 5a (a nitrogen heterocyclic carbene precursor)
[0063]
[0064] At room temperature, add triphenylmethane chloride 1 (5 g) and aniline (4.4 mL) to a 50 mL round-bottom flask in sequence. Heat to 190°C for 15 minutes. Cool to room temperature, add dilute hydrochloric acid (2M, 20 mL) and methanol (30 mL), heat to 80°C and stir for 30 minutes. Cool to room temperature, filter, wash with water, and dry. Suspend the dried solid in N,N-dimethylformamide (50 mL), cool to -15 °C, add concentrated sulfuric acid (5.5 mL), isoamyl nitrite (4 mL), stir for 1 hour, then Hypophosphorous acid (25 mL) was added dropwise. Slowly raise the temperature to 50°C and stir for 5 hours. Filtration and washing gave tetraphenylmethane 2 (5.2 g, 90%). Add liquid bromine (12 mL) into a 50 mL round bottom flask, add tetraphenylmethane 2 (4 g) in batches, stir at room temperature for 20 minutes, and cool to -78 ℃ , add...
Embodiment 2
[0068] Example 2, preparation of novel three-dimensional nitrogen heterocyclic carbene metal coordination assembly supported catalyst 6a:
[0069]
[0070] Under the protection of nitrogen atmosphere, rigid tetradentate imidazolium salt (azacyclic carbene precursor) 5a (0.58 g), [Ir(COD)Cl] 2(0.33 g), DMF (5 mL), tetrahydrofuran solution of LiHMDS (1M, 2 mL), stirred evenly and heated to reflux for 12 hours, a large amount of yellow solid precipitated, stop heating and cool the reaction system to room temperature. After adding 10 mL of distilled water to the reaction system, it was filtered, the solid was washed with distilled water, and dried in vacuo to obtain a brownish-yellow solid, which was the three-dimensional azacyclic carbene metal coordination-assembled catalyst 6a, with a yield of 0.70 g, 94%. The IR spectrum of product characterization is as attached Figure 4 shown.
[0071] IR (KBr pellet) ν 617.24, 682.62, 823.55, 1246.19, 1363.81, 1476.16, 1507.57, 155...
Embodiment 3
[0072] Example 3, preparation of novel three-dimensional nitrogen heterocyclic carbene metal coordination assembly supported catalyst 6b:
[0073]
[0074] Under the protection of nitrogen atmosphere, rigid tetradentate imidazolium salt (azacyclic carbene precursor) 5a (0.58 g), [Ir(CO) 2 (acac)] (0.35 g), DMF (5 mL), tetrahydrofuran solution of LiHMDS (1M, 2 mL), stirred evenly and heated to reflux for 12 hours, a large amount of brown-yellow solid precipitated, stop heating and cool the reaction system to room temperature . After adding 10 mL of distilled water to the reaction system, it was filtered, the solid was washed with distilled water, and dried in vacuo to obtain a brownish-yellow solid, which was the three-dimensional azacyclic carbene metal coordination-assembled supported catalyst 6b, with a yield of 0.68 g, 97%. The IR spectrum of product characterization is as attached Figure 5 shown.
[0075] IR (KBr pellet) ν 1363.72, 1477.25, 1507.74, 1544.43, 1559....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com