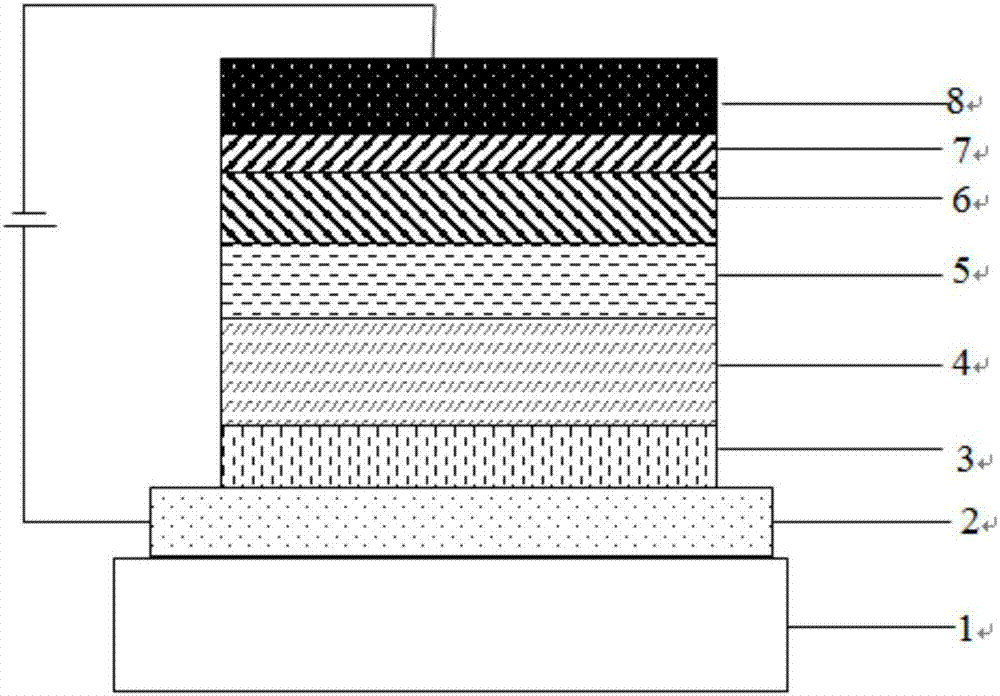
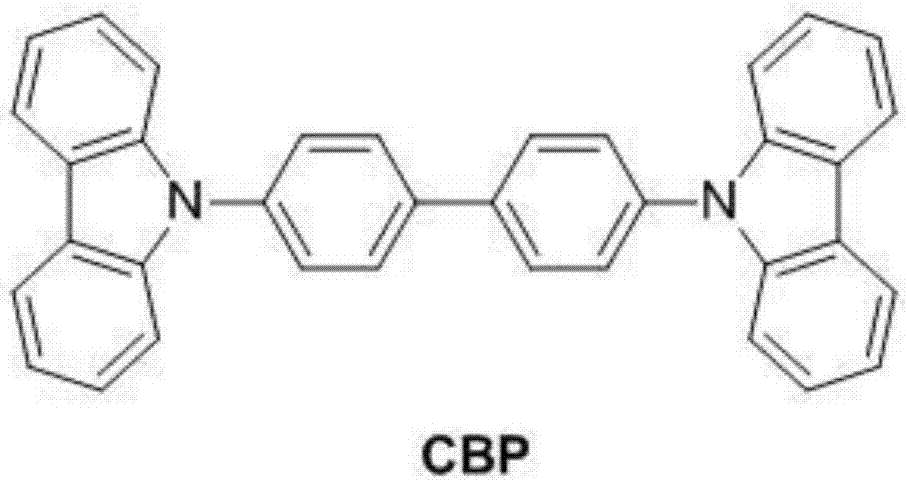
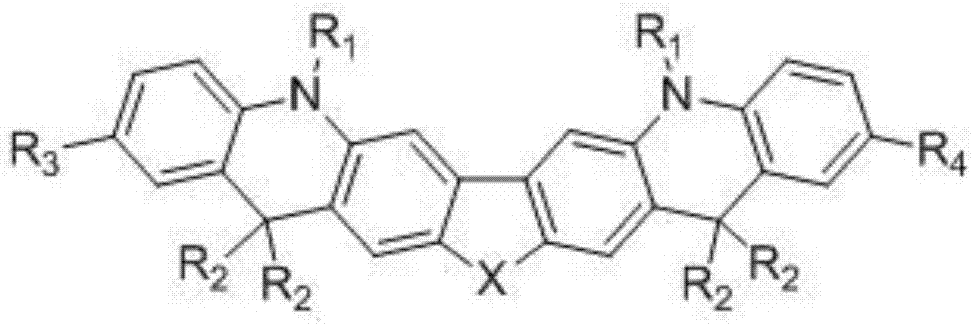
Organic electroluminescent material and application
An electroluminescent material and luminescent technology, applied in luminescent materials, electroluminescent light sources, organic light-emitting devices, etc., can solve problems such as the impact on the overall performance of the device, achieve improved device life, improved current efficiency, and increased spatial dimensions structure effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 compound C01
[0029]
[0030] Preparation of Compound 1: In a 1000mL three-neck flask, add 2,8-dibromodibenzofuran (32.6g, 0.1mol), aniline (18.6g, 0.2mol), sodium tert-butoxide (28.8g, 0.3mol) , xylene (750mL), palladium acetate (0.245g) and tri-tert-butylphosphine tetrafluoroborate (0.580g), under the protection of nitrogen, the temperature was raised to reflux, and the reaction was kept for 10h, and then cooled to room temperature, and added to the reaction flask 200mL deionized water, stirred for 15min, separated, washed the organic phase with 300mL deionized water for 3 times, collected the organic phase, anhydrous Na 2 SO 4 After drying, pass through a 50cm thick silica gel column, rinse the column with 1L of toluene, combine the column solution to remove the solvent, and use toluene:petroleum ether=1:6 Reflux beating and purification of the obtained yellow solid, after cooling, suction filtration and drying, to obtain compound...
Embodiment 2
[0036] The preparation of embodiment 2 compound C03
[0037]
[0038] Preparation of Compound 5: Dissolve compound C01 (11.7g, 0.02mol) in 100g dry DMF, transfer to a 250ml three-neck flask, under nitrogen protection, stir and dissolve at room temperature, slowly add NBS solid powder (7.1g, 0.04mol), kept at 120°C for 5 hours, the system quickly turned into a wine-red transparent system, after the heat preservation, it was naturally cooled to room temperature, added 200g of toluene and 150g of water, stirred for 0.5h, separated, collected the organic phase, and removed the solvent to obtain the compound The crude product of 5 was recrystallized using an equal proportion of toluene / ethanol as a solvent to obtain the fine product of compound 5, 10.6 g of light yellow solid, yield 71.6%, MS (m / s): 740.1;
[0039] Preparation of compound C03: In a 250mL three-necked flask, add compound 5 (7.41g, 0.01mol), phenylboronic acid (2.67g, 0.022mol), potassium carbonate (3.45g, 0.025mo...
Embodiment 3
[0041] The preparation of embodiment 3 compound C04
[0042]
[0043] Preparation of compound C04: In a 500mL three-necked flask, add tert-butyl chloride (0.93g, 0.01mol), chlorobenzene (70g), under the protection of nitrogen, the system is lowered to 5-10°C, and compound C01 is added dropwise to the reaction flask (5.83g, 0.01mol) and chlorobenzene (200g) solution, keep warm at 30-50°C for 6-8h after dropping; after the reaction, pour the reaction solution into dilute hydrochloric acid, separate the liquid and collect the organic phase, Na 2 SO 4 After drying, filtering, and removing the solvent, the crude product was purified by silica gel column chromatography, and the eluent was toluene:petroleum ether=1:3 to obtain compound C04, 4.9 g of light yellow solid, with a yield of 76.9%.
[0044] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 46 h 42 N 2 O, theoretical value 638.3297, test value 638.3159. Elemental analysis (C 46 h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com