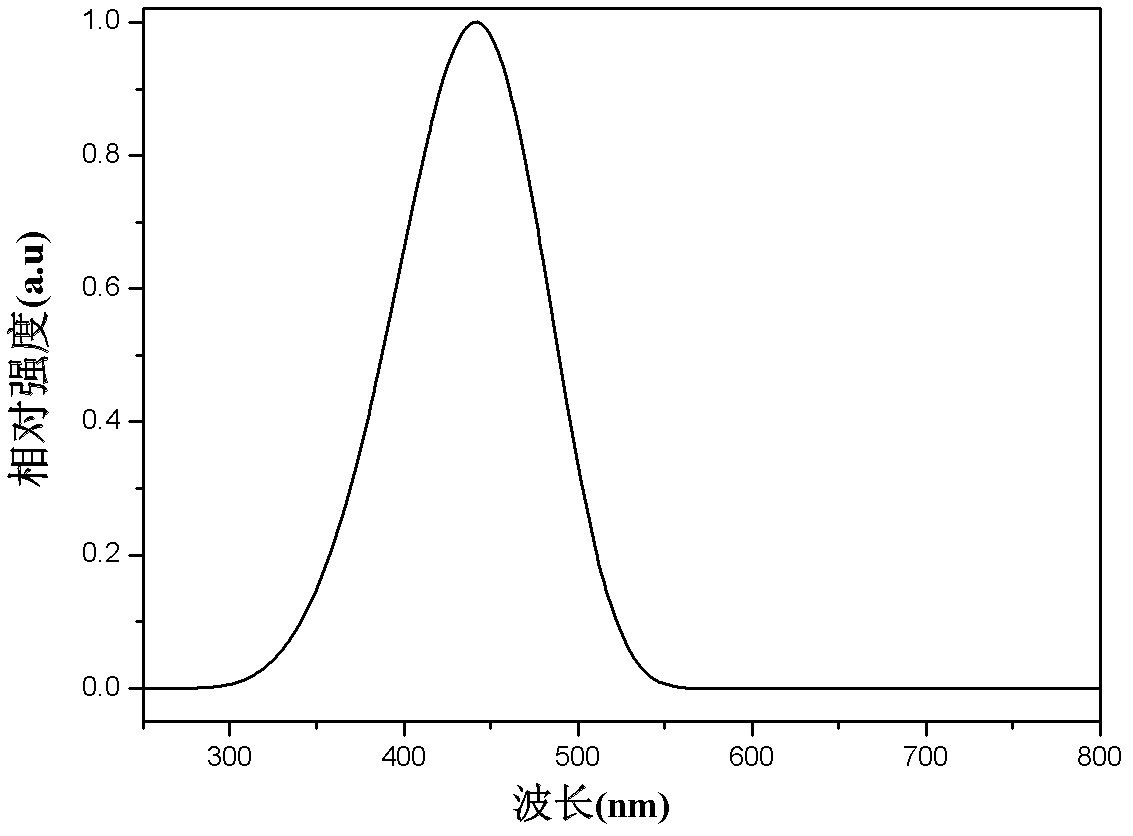
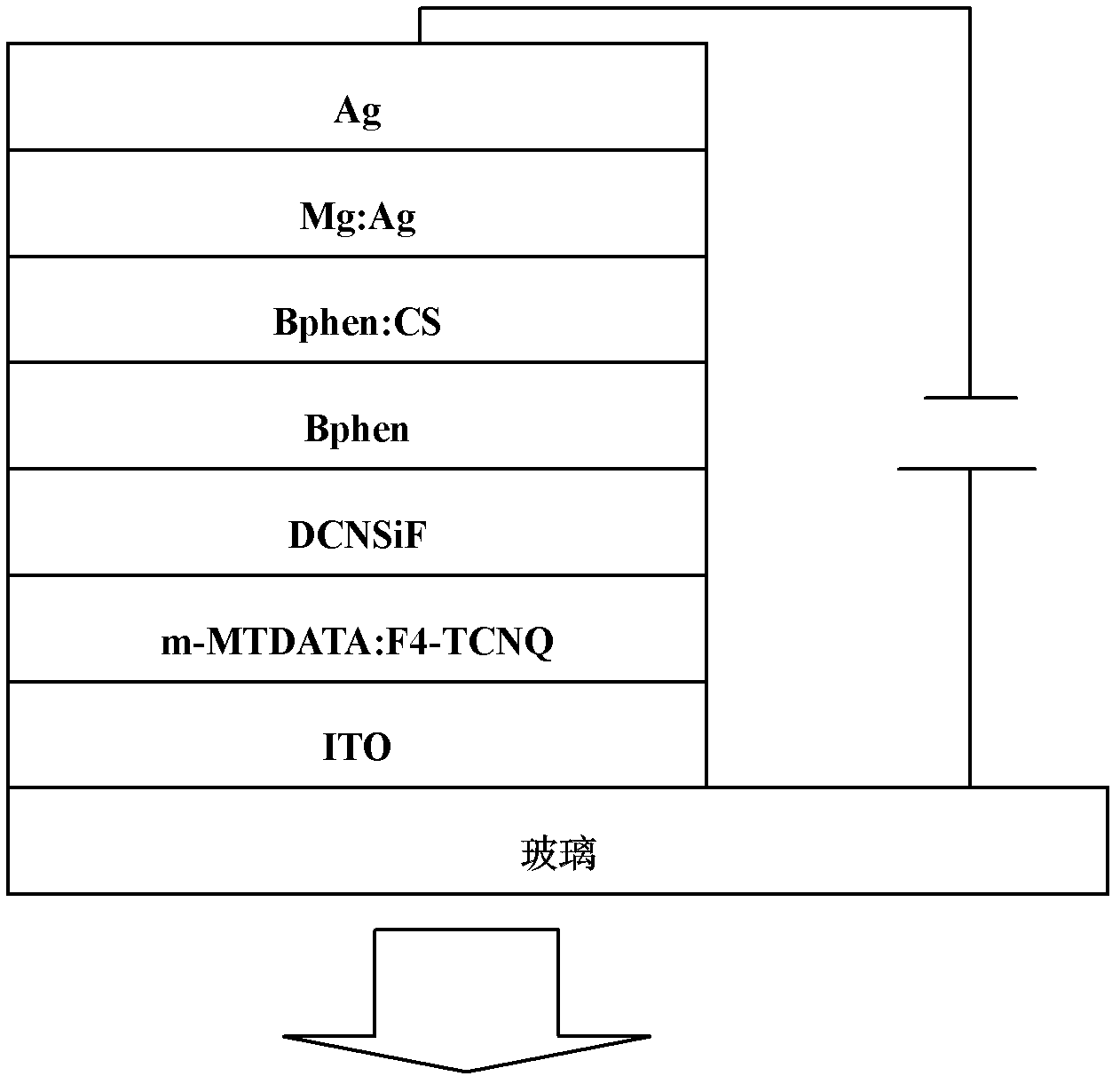
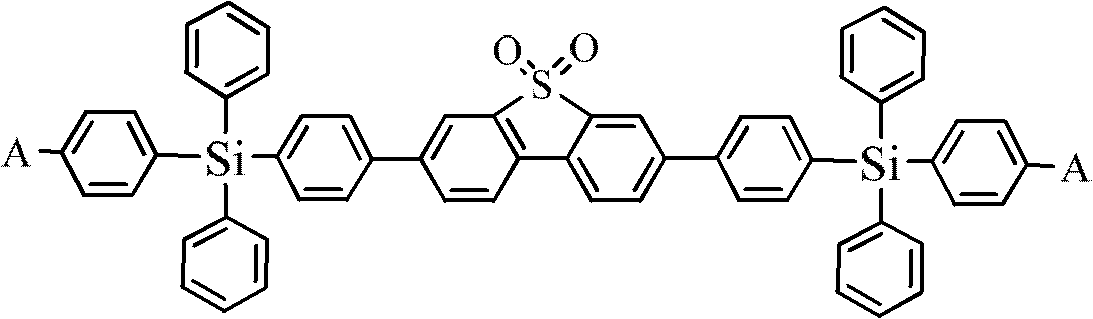
Dibenzothiophene sulphone containing organic semiconductor material and preparation method thereof and organic electroluminescent device
A technology of organic semiconductor and thiophene sulfone, applied in the field of organic electroluminescent devices, can solve the problems of high mobility, lack of high thermal stability blue light materials, etc., and achieve the effects of excellent thermal stability, convenient operation and good electron transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the above-mentioned organic semiconductor material containing dibenzothiophene sulfone, the steps are as follows:
[0028] S1, provide the following compounds:
[0029] Compound I: Compound II: or Among them, in the compound formula II, A- is a fluorine atom (-F), a cyano group (-CN) or a nitro group (-NO 2 any of );
[0030] S2. In an inert gas atmosphere (including nitrogen or argon, etc.), dissolve compounds I and II in an organic solvent containing a catalyst and an alkali solution at a molar ratio of 1:2 to 1:3, and heat to reflux at 70 to 110°C Carry out the Suzuki reaction under the condition for 20~48h, the obtained structural formula is The compound, namely ASiF; the reaction formula is as follows:
[0031]
[0032] Wherein, in step S2 of the above-mentioned preparation method, the catalyst is organic palladium (such as tetrakis(triphenylphosphine) palladium (Pd(PPh 3 ) 4 ), tris(dibenzylideneacetone)dipalladium (Pd 2 (...
Embodiment 1
[0043] This embodiment discloses a dibenzothiophene sulfone-containing organic semiconductor material with the following structural formula, namely 2,7-bis(4-((4-cyanophenyl)diphenylsilyl)phenyl)thiophene (DCNSiF)
[0044]
[0045] Preparation of 2,7-bis(4-((4-cyanophenyl)diphenylsilyl)phenyl)thiophene (DCNSiF):
[0046]
[0047]Add 3mmol of 2,7-dibromothiophene, 6.0mmol of 4-((4-cyanophenyl)diphenylsilyl)phenylboronic acid, and 0.003mmol of tetrakis(triphenylphosphine)palladium into the reaction flask, vacuumize, After circulating nitrogen for 3 times, the reaction system was in an oxygen-free state. Under the protection of nitrogen, 50 mL of tetrahydrofuran and 2 mol / L Na 2 CO 3 Aqueous solution 35ml, the mixture was heated to 70 ° C reflux Suzuki reaction 48h.
[0048] After the reaction, the reaction solution was poured into saturated ammonium chloride aqueous solution, extracted three times with dichloromethane, the organic phase was washed with sodium chloride aq...
Embodiment 2
[0052] This embodiment discloses a dibenzothiophene sulfone-containing organic semiconductor material having the following structural formula, that is, 2,7-bis(4-((4-fluorophenyl)diphenylsilyl)phenyl)thiophene (DFSiF)
[0053]
[0054] Preparation of 2,7-bis(4-((4-fluorophenyl)diphenylsilyl)phenyl)thiophene (DFSiF):
[0055]
[0056] Add 3 mmol of 2,7-dibromothiophene, 6.4 mmol of 4-((4-fluorophenyl)diphenylsilyl)phenylboronate, and 0.09 mmol of bis(triphenylphosphine)palladium dichloride to the reaction flask , after evacuating and circulating argon for 3 times, the reaction system was in an oxygen-free state. Under the protection of argon, 50 mL of ethylene glycol dimethyl ether and 2 mol / L of Cs were added. 2 CO 3 Aqueous solution 40ml, the mixture was heated to 90 ℃ reflux Suzuki reaction for 36h.
[0057] After the reaction, the reaction solution was poured into saturated ammonium chloride aqueous solution, extracted three times with dichloromethane, the organic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com