Antiviral composition containing glucosan
A dextran and anti-virus technology, which is applied in the direction of disinfectant, biocide, animal repellent, etc., can solve the problems that the compounding technology of dextran and component A has not been reported, and achieve the goal of field application The effect of reducing quantity, reducing single selection pressure, and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 11% dextran · Component A emulsifiable concentrate
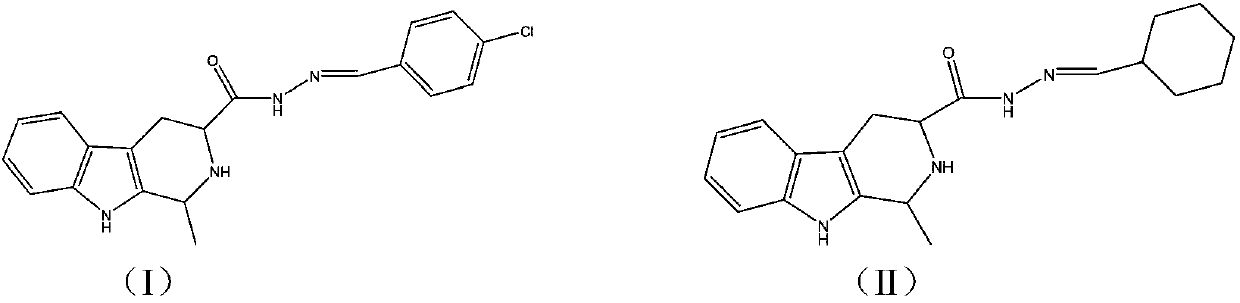
[0033] Raw material composition: 1% dextran sugar, 10% component A technical drug, 5% N-methylpyrrolidone, 3% calcium dodecylbenzenesulfonate, 2% fatty alcohol ether sodium sulfate phosphate, 5% benzene Ethylphenol formaldehyde resin polyoxyethylene ether, and the rest is made up with aromatic hydrocarbon S-150. Prepared according to the conventional cream production method. The component A is (1S,3S)-N'-(4-chlorophenylmethylene)-1-methyl-2,3,4,9-tetrahydropyrido[3,4-b ] indole-3-carboxyl hydrazide.
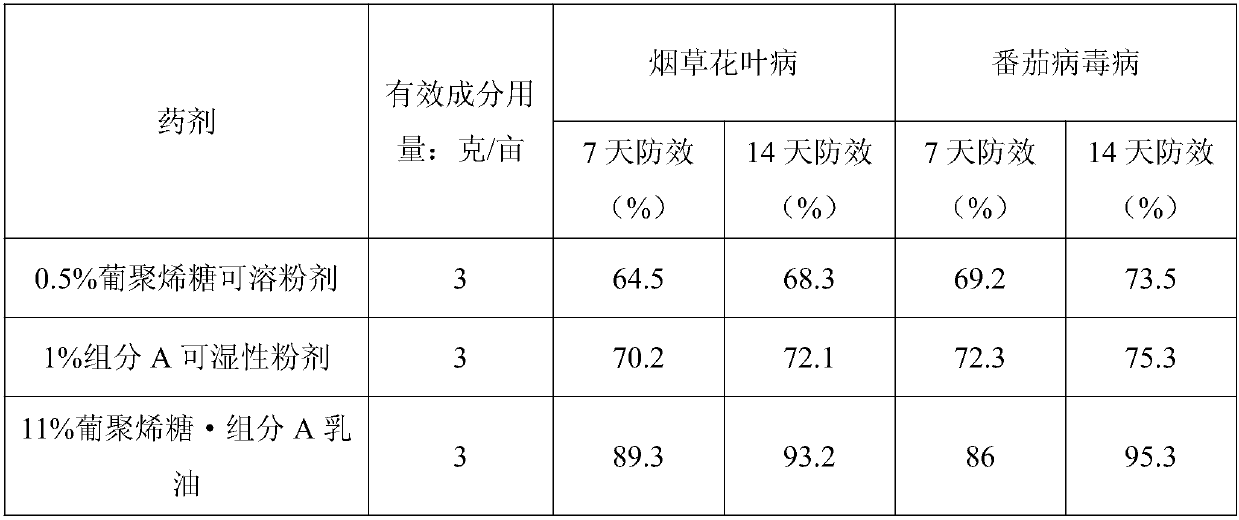
[0034] Example 1 sample, the control agent is 0.5% dextran sugar soluble powder (commercially available) and 1% component A wettable powder (laboratory production) for comparative drug efficacy comparison test. Control objects: Tobacco mosaic disease, tomato virus disease.
[0035]
[0036] The average temperature during medication is 21-25°C, and the weather is mainly sunny. The data show that the anti-bacterial ...
Embodiment 2
[0038] 30% Dextran · Component A Suspending Concentrate
[0039] The raw material composition is: 20% dextran, 10% component A, 1% sodium lignin sulfate, 2% calcium dodecylbenzenesulfonate, 3% tristyryl phenol polyoxyethylene ether, fatty alcohol 1.0% polyoxyethylene ether, 1% magnesium aluminum silicate, 0.10% xanthan gum, 5% ethylene glycol, and make up the rest with water. Prepared according to the conventional production method of suspension concentrate. The component A is (1S,3S)-N'-(cyclohexylmethylene)-1-methyl-2,3,4,9-tetrahydropyrido[3,4-b]indole -3-Formohydrazide.
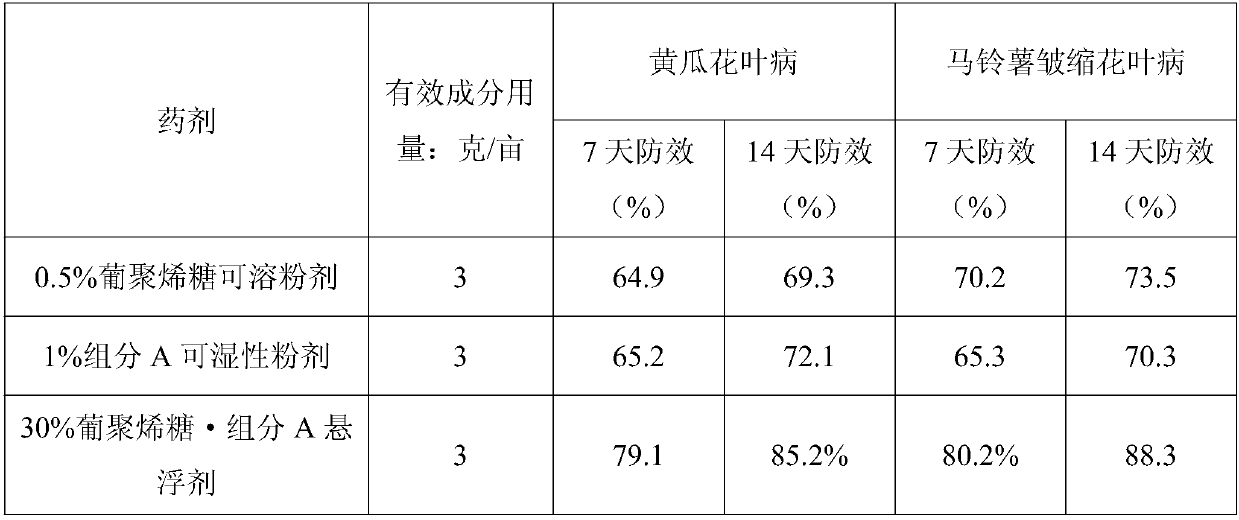
[0040] The sample of Example 2, the control agent is 0.5% dextran sugar soluble powder (commercially available) and 1% component A wettable powder (laboratory production) for comparative drug efficacy comparison test. Control objects: cucumber mosaic disease, potato shrunken mosaic disease.
[0041]
[0042] The average temperature during medication is 21-25°C, and the weather is mainly sunny. The da...
Embodiment 3
[0044] 41% dextran · component A wettable powder
[0045] The raw material composition is 1% dextran, 40% component A, 1% sodium lauryl sulfate, 7% sodium alkylphenol ethoxylate sulfonate, 2% sodium lignosulfonate, 3.5% lignin Sulfonate dispersant, the rest is supplemented with light calcium carbonate. Formulated according to conventional wettable powder production methods. The component A is (1S,3S)-N'-(cyclohexylmethylene)-1-methyl-2,3,4,9-tetrahydropyrido[3,4-b]indole -3-Formohydrazide.
[0046] The sample of Example 3, the control agent is 0.5% dextran sugar soluble powder (commercially available) and 1% component A wettable powder (laboratory production) for comparative drug efficacy comparison test. Control objects: turnip mosaic disease, potato shrunken mosaic disease.
[0047]
[0048] The average temperature during medication is 21-25°C, and the weather is mainly sunny. The data show that the anti-bacterial efficiency of the compound preparation is improved com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com