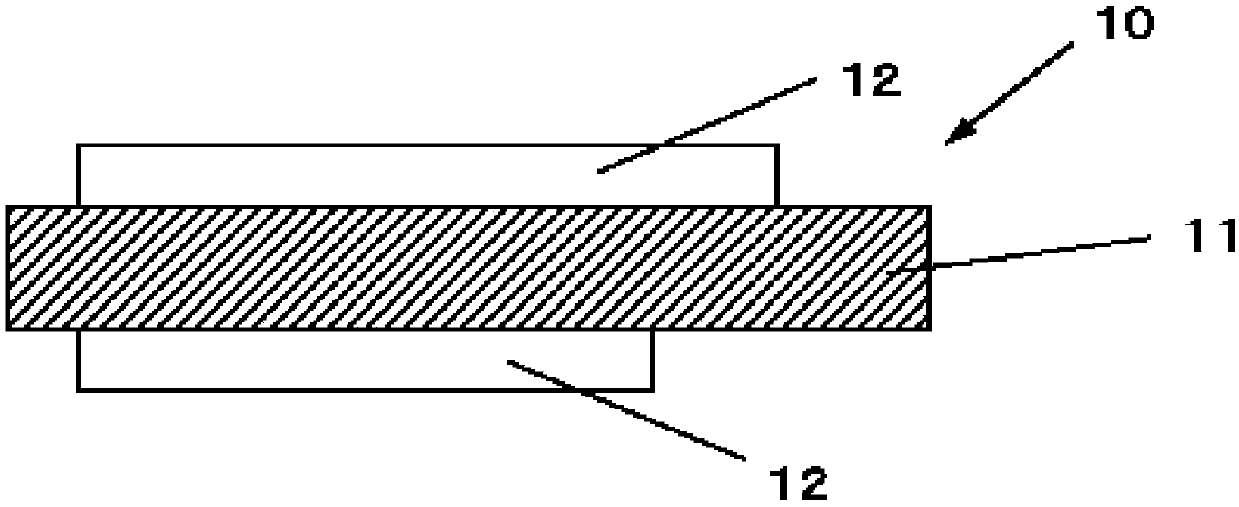
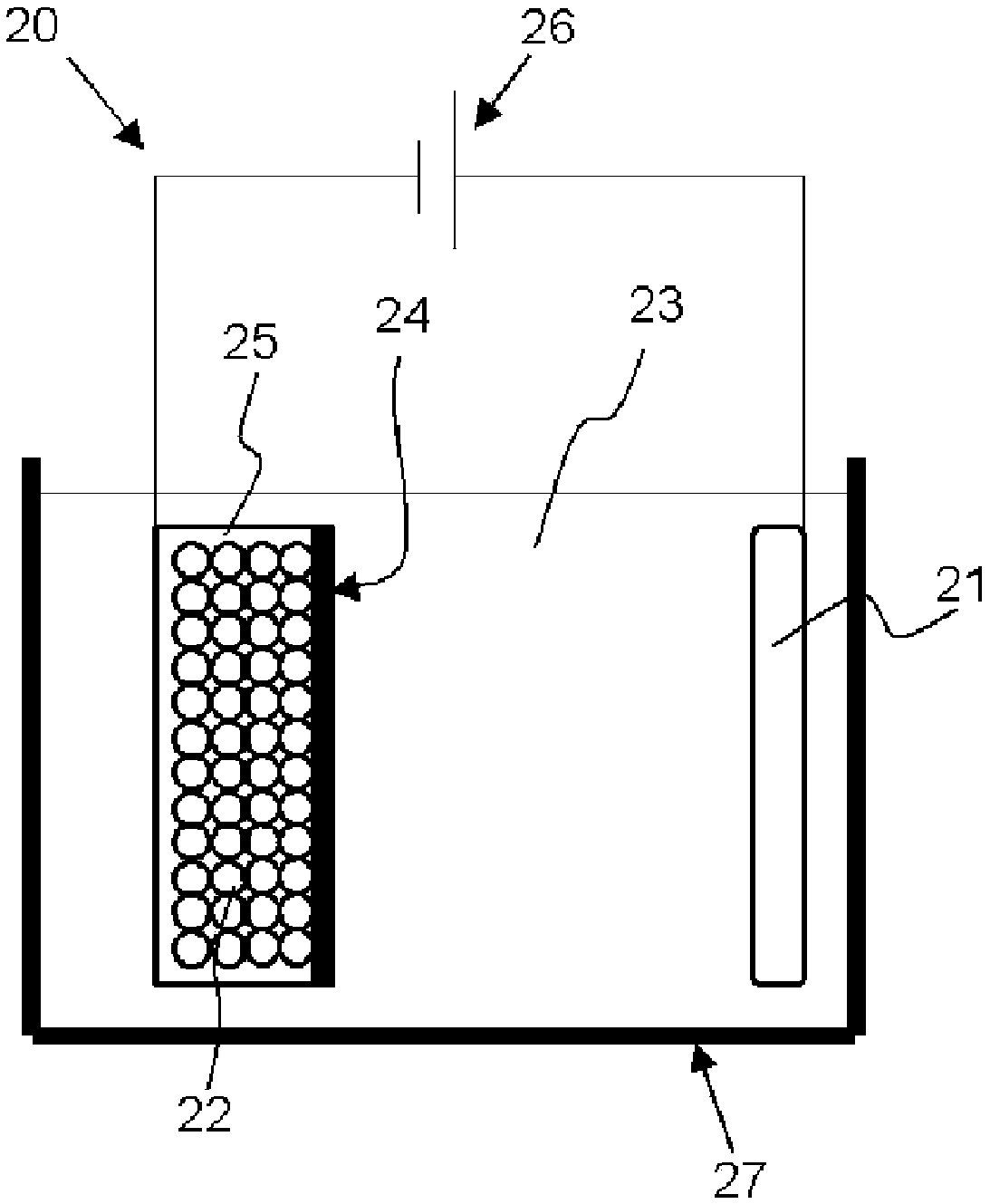
Negative electrode active material for non-aqueous electrolyte secondary cell, negative electrode for non-aqueous electrolyte secondary cell, non-aqueous electrolyte secondary cell, and method for manufacturing negative electrode active material particles
一种负极活性物质、非水电解质的技术,应用在非水电解质蓄电池、活性物质电极、二次电池等方向,能够解决耗费电解液、循环特性降低等问题,达到抑制分解、抑制导电性下降、提高容量维持率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
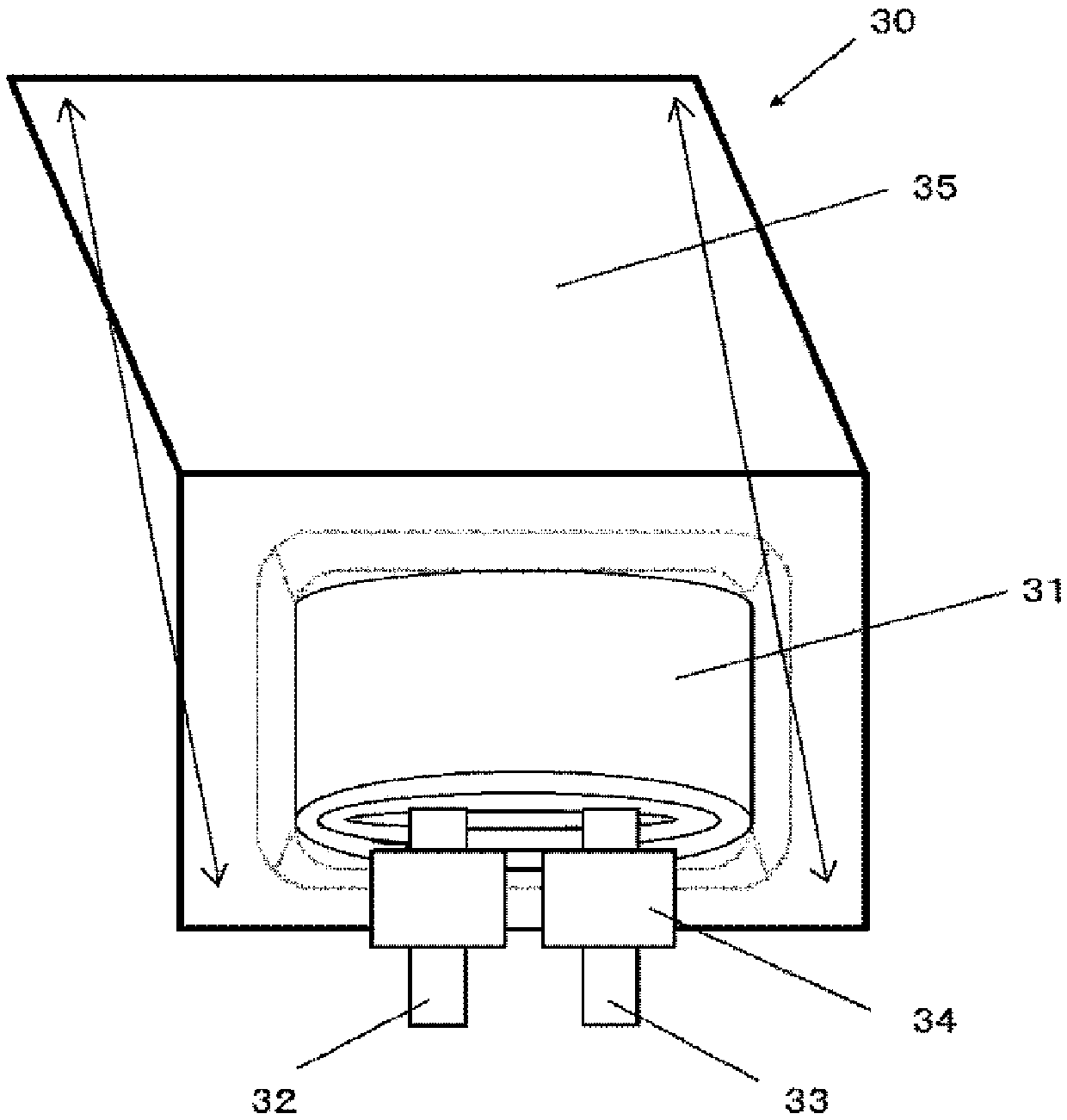
[0195] According to the following sequence, make as image 3 A laminated film type secondary battery 30 is shown.
[0196] Make the positive electrode first. The positive electrode active material is a mixed lithium-cobalt composite oxide, namely LiCoO 2 95 parts by mass, 2.5 parts by mass of positive electrode conductive aid, and 2.5 parts by mass of positive electrode binder (polyvinylidene fluoride, PVDF) to prepare a positive electrode mixture. Next, the positive electrode mixture is dispersed in an organic solvent (N-methyl-2-pyrrolidone, NMP) to form a pasty slurry. Next, the slurry was coated on both sides of the positive electrode current collector with a coating device having a die, and dried with a hot air drying device. At this time, the thickness of the positive electrode current collector was 15 μm. Finally, compression molding is performed by rolling.
[0197] Next, make the negative electrode. In order to make the negative electrode, the raw material (als...
Embodiment 1-2~1-5
[0204] (Examples 1-2 to 1-5, Comparative Example 1-1, Comparative Example 1-2)
[0205] A secondary battery was produced in the same manner as in Example 1-1 except for adjusting the amount of oxygen in the bulk of the silicon compound. At this time, the amount of oxygen is adjusted by changing the ratio and temperature of the vaporized starting material. Table 1 shows x values of silicon compounds represented by SiOx in Examples 1-1 to 1-5, Comparative Example 1-1, and Comparative Example 1-2.
[0206] After checking the cycle characteristics (maintenance rate %) and the initial charge and discharge characteristics (initial efficiency %) of the secondary batteries of Examples 1-1 to 1-5, Comparative Example 1-1, and Comparative Example 1-2, the results shown in Table 1 were obtained. the results shown.
[0207]The cycle characteristics were checked as follows. First, in order to stabilize the battery, charge and discharge were performed twice in an environment of 25°C, a...
Embodiment 2-1~ Embodiment 2-6、 comparative example 2-1
[0218] A secondary battery was manufactured basically in the same manner as in Examples 1-3, but in the silicon compound represented by SiOx, the conditions of lithium doping treatment (in-bulk modification), that is, the treatment conditions of lithium doping, were changed to Change the type of lithium compound included. After examining the cycle characteristics and initial charge and discharge characteristics of the secondary batteries of Examples 2-1 to 2-6 and Comparative Example 2-1, the results shown in Table 2 were obtained.
[0219] [Table 2]
[0220] SiOx (x=0.9); D 50 =5.2μm; XRD half value width 2θ=1.85°; Si(111) crystallite 4.62nm; I1330 / I1580=1.2;
[0221] Carbon content: 5% by mass; carbon layer thickness: 100nm; carbon layer coverage: 80%;
[0222] Phosphorus content: 2000 mass ppm; boron content: 0 mass ppm; sodium content: 20 mass ppm; potassium content: 30 mass ppm; magnesium content: 25 mass ppm; calcium content: 30 mass ppm; aluminum content: 250 mass pp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com