Carbon nitride and graphene composite material modified by organic dye and application of carbon nitride and graphene composite material
A technology of organic dyes and composite materials, applied in organic dye-modified carbon nitride graphene composite materials and its application fields, can solve the problems of small specific surface area and low quantum efficiency of graphite phase carbon nitride, and achieve simple and easy preparation methods Line, simple equipment, high effect than the surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1. g-C 3 N 4 preparation
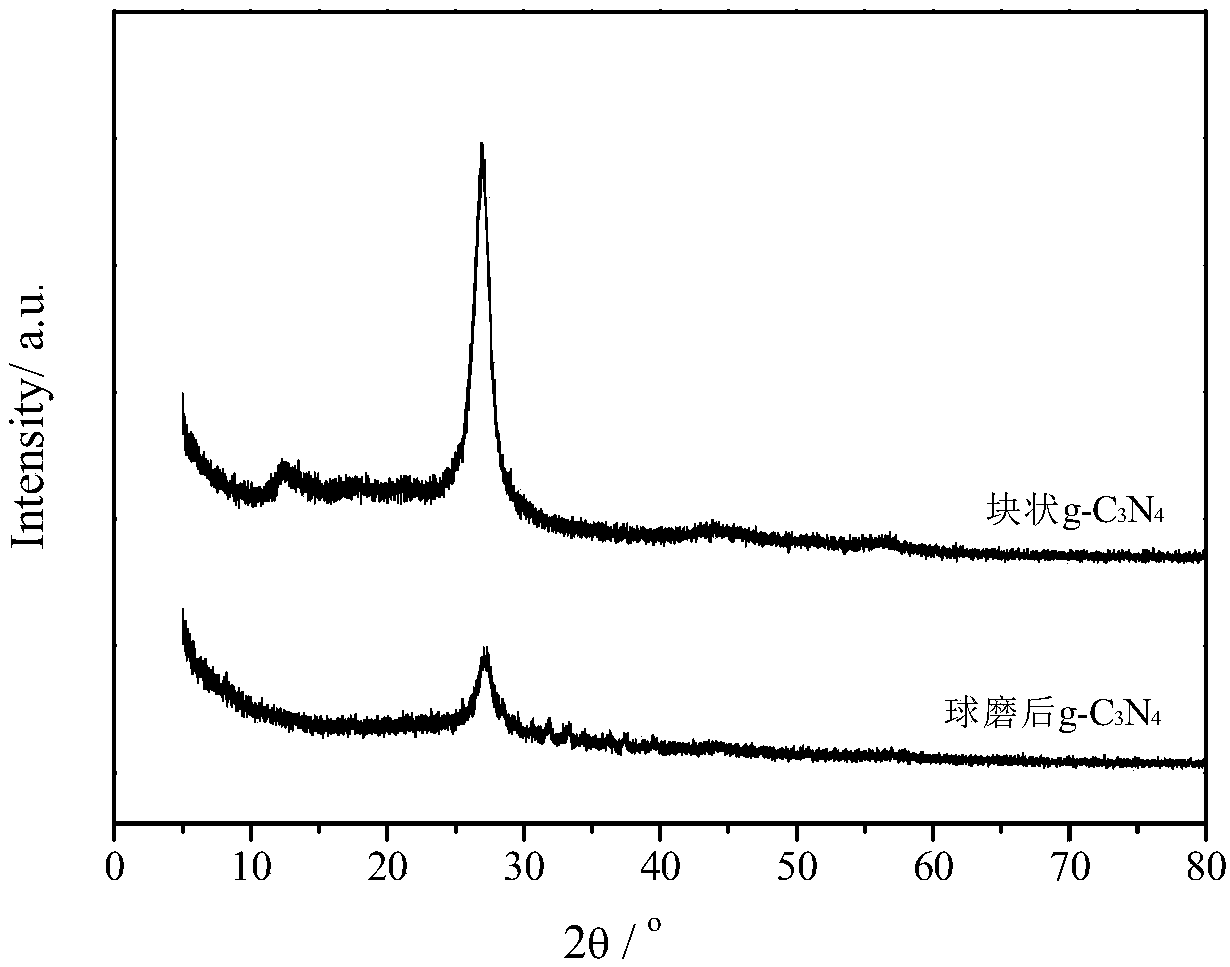
[0030] Take 20g of dipolycyanamide into a 50ml crucible, cover it with a lid, put it into a muffle furnace, raise the temperature from room temperature to 550°C at a heating rate of 2.2°C, keep the temperature at 550°C for 4 hours, and cool down to room temperature to obtain a yellow color Sample block g-C 3 N 4 8g, grind into powder with a mortar and set aside, for g-C 3 N 4 Samples were characterized by X-ray diffraction ( figure 1 ), the characteristic peaks 003 and 002 appear at 13.3° and 27.1°, respectively.
[0031] 2. g-C 3 N 4 Ball milling pretreatment:
[0032] 1.0g of g-C 3 N 4 The powder and 100 mL of 2mol / L NaOH aqueous solution were loaded into a ball mill bowl with steel balls with a diameter of 8 mm, and the steel balls were mixed with g-C 3 N 4 The mass ratio is 50:1, the rotational speed is 100rpm, and the ball mill is 72 hours. The steel balls are separated to obtain a black suspension, and the black precipitat...
Embodiment 2
[0037] 1. g-C 3 N 4 Ball milling pretreatment:
[0038] 1.0 g of g-C prepared in Example 1 3 N 4 The powder and 100 mL, 3 mol / L KOH aqueous solution were loaded into a ball mill bowl with steel balls with a diameter of 8 mm, and the steel balls were mixed with g-C 3 N 4 The mass ratio is 400:1, the rotational speed is 600rpm, ball milling for 2 hours, the steel balls are separated to obtain a black suspension, 3000rpm centrifugation to obtain a black precipitate, add 100ml, 0.5mol / L hydrochloric acid aqueous solution and stir magnetically at 90°C for 72h to remove the impurity Fe, Then centrifuged and washed repeatedly with deionized water until pH = 7, and the precipitate was freeze-dried at -50°C for 72 hours to obtain 0.7 g of nitrogen yellow product, which was g-C after ball milling. 3 N 4 . The resulting product was characterized by X-ray diffraction, and with block g-C 3 N 4 For comparison, it can be seen that the 002 peak of the sample after ball milling decrea...
Embodiment 3
[0042] 1. g-C 3 N 4 Ball milling pretreatment:
[0043] 1.0 g of g-C prepared in Example 1 3 N 4 The powder and 100 mL of 1 mol / L NaOH aqueous solution were loaded into a ball mill bowl with steel balls with a diameter of 8 mm, and the steel balls were mixed with g-C 3 N 4 The mass ratio is 200:1, the speed is 400rpm, ball milling is 24 hours, the steel balls are separated to obtain a black suspension, 3000rpm is centrifuged to obtain a black precipitate, and 100ml of 0.5mol / L hydrochloric acid solution is added to magnetically stir at 50°C for 48h to remove the impurity Fe, and then Centrifuge and wash repeatedly with deionized water until pH = 7, and obtain a precipitate. Freeze-dry at -55°C for 48 hours to obtain 0.9 g of nitrogen yellow product, which is g-C after ball milling. 3 N 4 . The resulting product was characterized by X-ray diffraction, and the bulk g-C 3 N 4 For comparison, it can be seen that the 002 peak of the sample after ball milling decreased sign...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com