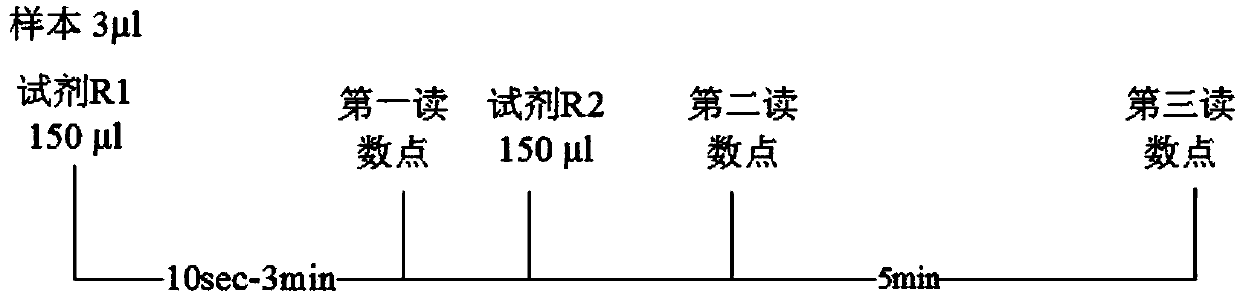
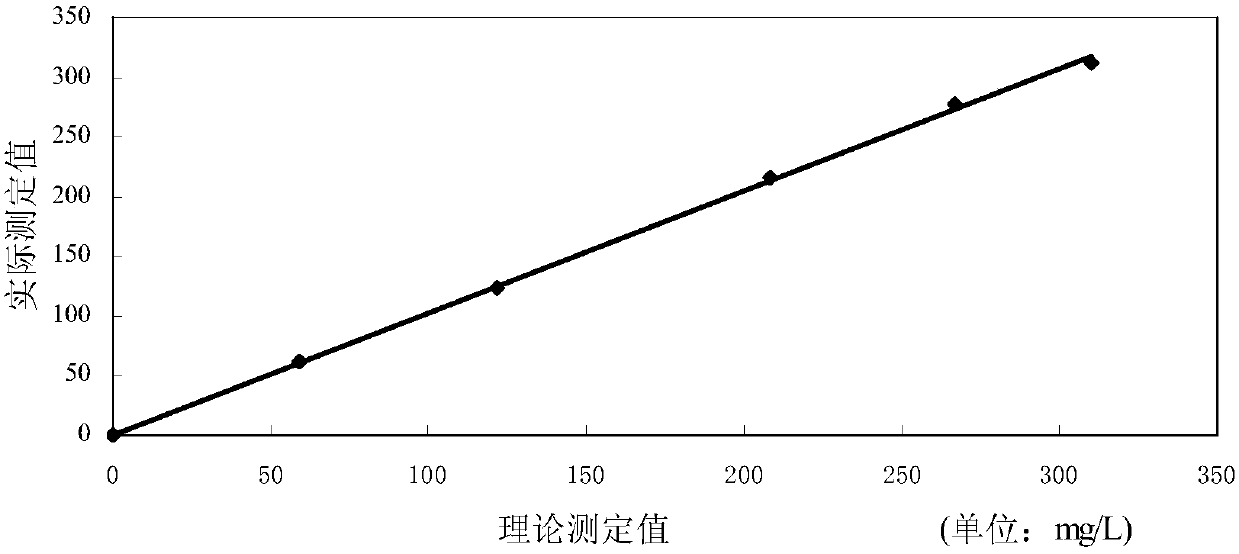
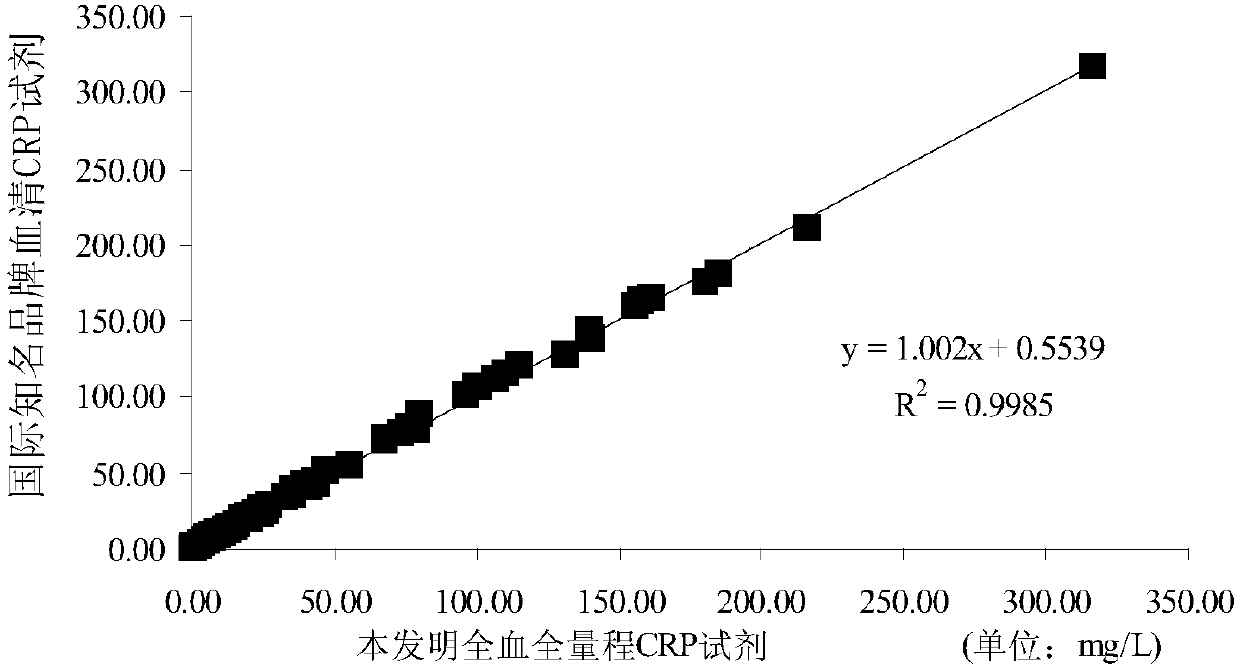
Reagent and method for quantitatively detecting full-scale C-reactive protein in whole blood
A technology of reactive protein and detection reagents, which is applied in the field of detection reagents, can solve the problems of not mentioning the detection of full-scale CRP, and the absence of quantitative detection reagents for full-scale CRP in whole blood, etc., to achieve a wide range of clinical applications, less sample demand, Apply a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A full range of C-reactive protein detection reagents in whole blood, including reagent R1 and reagent R2.
[0043] Specifically:
[0044] Reagent R1 includes: cell-breaking components, buffer, surfactant, electrolyte, preservative, reaction accelerator, and stabilizer. Wherein: the cell-breaking component is a fatty acid salt with a mass percentage of 0.05%, the buffer solution is a glycine buffer solution with a pH value of 5.0 and a concentration of 30mmol / L, the surfactant is Tween20 with a mass percentage of 0.01%, and the electrolyte is a mass percentage The sodium salt is 0.1%, the preservative is sodium azide with a mass percentage of 0.15%, the reaction accelerator is polyethylene glycol 2000 with a mass percentage of 0.20%, and the stabilizer is bovine serum albumin with a mass percentage of 0.50%.
[0045] Reagent R2 includes: anti-human C-reactive protein sensitized latex particles, surfactants, stabilizers, preservatives, electrolytes, and buffers. Wherei...
Embodiment 2
[0050] A full range of C-reactive protein detection reagents in whole blood, including reagent R1 and reagent R2.
[0051] Specifically:
[0052] Reagent R1 includes: cell-breaking components, buffer, surfactant, electrolyte, preservative, reaction accelerator, and stabilizer. Wherein: the broken cell component is the sulfate ester salt of 1.50% by mass percent, the buffer solution is the Tris buffer solution with a pH value of 10.0 and a concentration of 50mmol / L, and the surfactant is Emulgen B-66 with 3.00% by mass percent, The electrolyte is 1.5% potassium salt by mass percentage, the preservative is 2.00% by mass sorbate, the reaction accelerator is 3.00% by mass of polyethylene glycol 4000, and the stabilizer is 2.00% by mass of gelatin.
[0053] Reagent R2 includes: anti-human C-reactive protein sensitized latex particles, surfactants, stabilizers, preservatives, electrolytes, and buffers. Wherein: the mixing ratio of large latex particles and small latex particles i...
Embodiment 3
[0058] A full range of C-reactive protein detection reagents in whole blood, including reagent R1 and reagent R2.
[0059] Specifically:
[0060] Reagent R1 includes: cell-breaking components, buffer, surfactant, electrolyte, preservative, reaction accelerator, and stabilizer. Wherein: the broken cell component is the phosphate ester salt of 0.50% by mass percentage, the buffer solution is the phosphoric acid-citric acid buffer solution with a pH value of 7.0 and a concentration of 50mmol / L, and the surfactant is Span20 with 1.00% by mass percentage, The electrolyte is 1.00% by mass percent sodium salt, the preservative is 1.00% by mass percent gentamicin, the reaction accelerator is 1.00% by mass percent polyethylene glycol 8000, and the stabilizer is 1.00% by mass percent of ethylene glycol.
[0061] Reagent R2 includes: anti-human C-reactive protein sensitized latex particles, surfactants, stabilizers, preservatives, electrolytes, and buffers. Among them: the mixing rati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com