A kind of cataplasm of total coumarin cataplasm for improving the bioavailability of ancestralin
A technology of Zushi ephedrin and total coumarin, which is applied in anti-inflammatory agents, drug combinations, drug delivery, etc., can solve the problems of large fluctuations in blood drug concentration, low bioavailability, and short half-life, and achieve improved Bioavailability, the effect of achieving transdermal absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
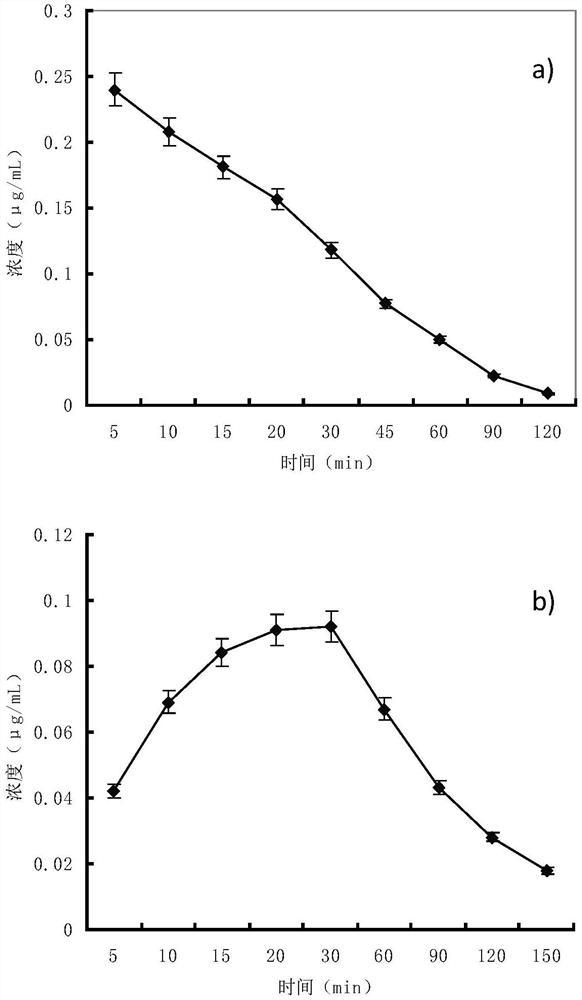
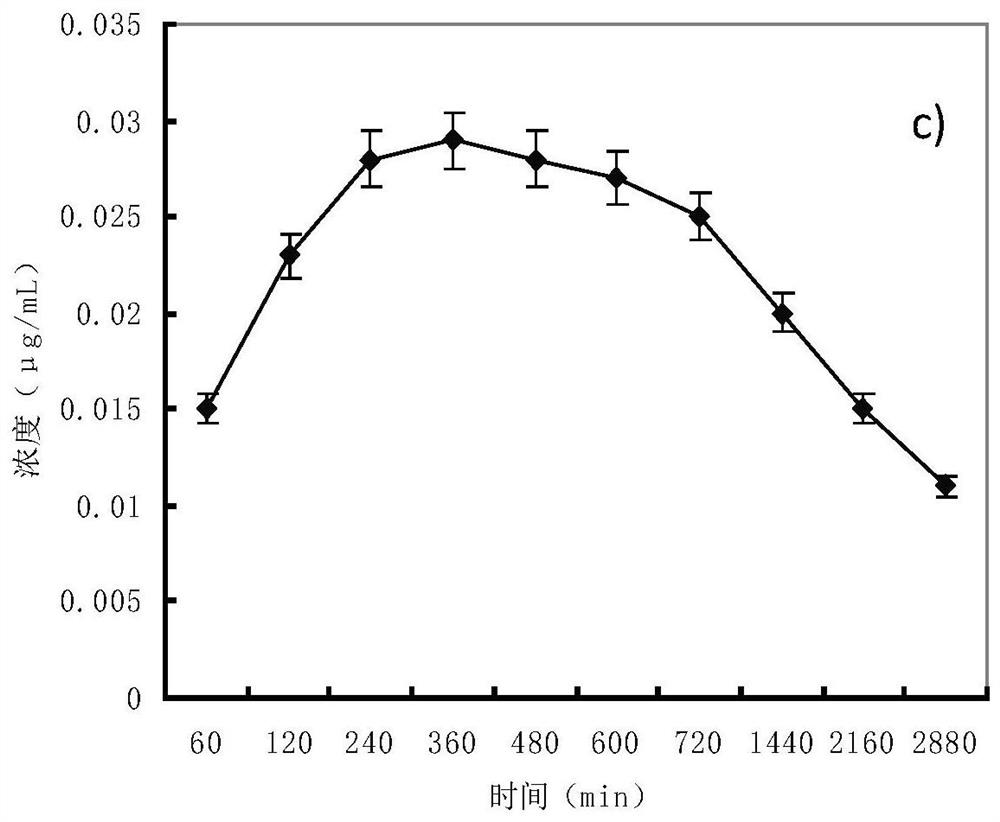
Examples
Embodiment 1
[0023] Example 1 Preparation of Zushi total coumarin.
[0024] Extract Zushima with 12 volume times of 60% (v / v) ethanol by percolation at 3 mL / min / kg for 12 h, collect the percolation liquid, recover ethanol, concentrate until there is no alcohol smell, add water to disperse to a concentration of 0.05g / mL, centrifuge at 3000r / min for 20 minutes, suspend the precipitate with 2 times the weight of hot water, centrifuge again, combine the centrifugates twice, adjust the pH to 3.0 with 1mol / L hydrochloric acid, load the sample at 6 times the column volume, pass D-101 macroporous resin column (diameter-to-height ratio of the column is 1:5), the flow rate is 2 times the column volume per hour, first elute with 4 times the column volume of water, discard the water eluate, and then wash it with 5 times the column volume The 50% ethanol of the column volume is eluted, and the flow rate of the eluent is still 2 times of the column volume per hour. Collect 50% ethanol eluate, recover ...
Embodiment 2
[0025] Example 2 Preparation of Zushima total coumarin cataplasm containing Zushima A.
[0026] 2.1 Preparation of cataplasm
[0027] (1) Take 32g of glycerol, add 5.5g of sodium polyacrylate NP700, 0.14g of glycerol, and 0.12g of disodium ethylenediaminetetraacetate in sequence, stir and mix well to obtain solution I;
[0028] (2) Take 45g of deionized water, add 0.934g of the total coumarin extract of Zumima and 2.51g of azone in sequence in Example 1, and ultrasonicate until uniformly dispersed to obtain solution II;
[0029] (3) Slowly add solution II to solution I, stir while adding, and stir well to obtain a paste;
[0030] (4) Apply the paste to the non-woven fabric with a coating machine, so that every 100cm 2 The paste content of the non-woven fabric is 6g, and the total coumarin extract content of each gram of the paste is 10.84mg. Let it dry for a while, and then the cataplasm S1 is obtained.
[0031] 2.2 Preparation of cataplasm
[0032] (1) Take 35g of glycero...
Embodiment 3
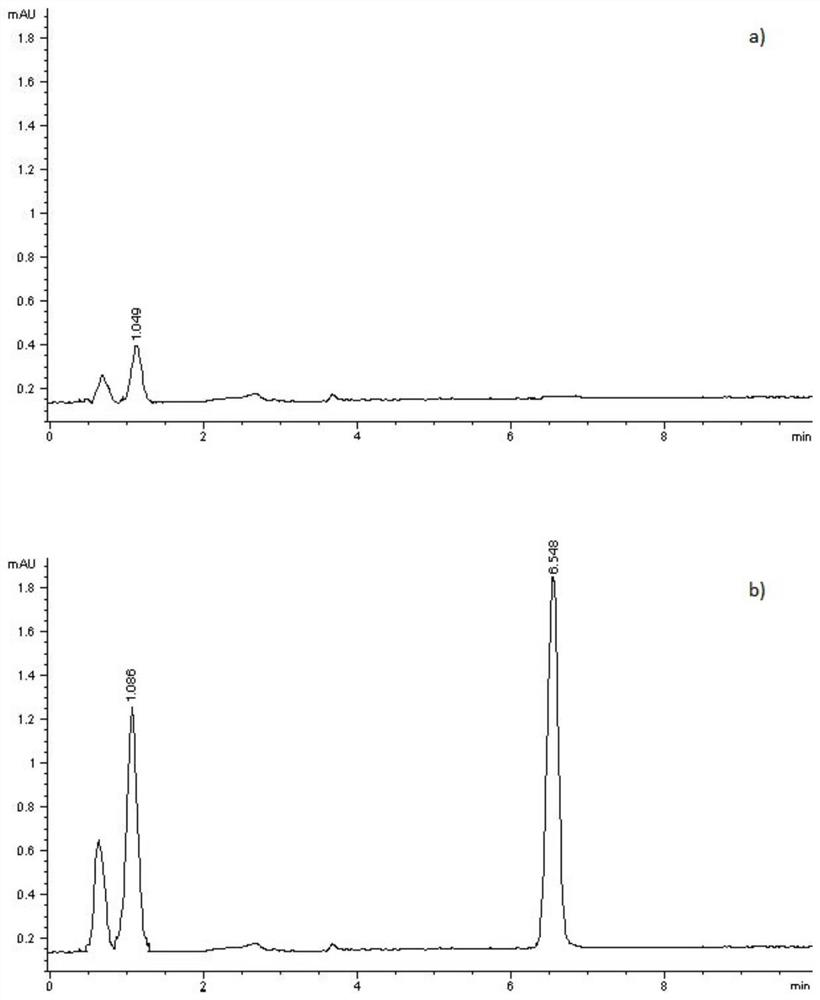
[0046] Example 3 Evaluation of the adhesion and appearance of cataplasms.
[0047] The cataplasms of each formulation in Example 2 were evaluated for adhesion and appearance. The measurement method is as follows, and the measurement results are shown in Table 1.
[0048] 3.1 Determination of adhesion force
[0049] Take 5 pieces of shaped pap plaster (area 5cm×10cm), remove the anti-adhesive layer, paste them on a clean phenolic resin board, roll them back and forth twice with a 2kg roller, leave them for 20min, uncover one end by 1cm, and clamp them with The pointer push-pull force gauge is 180° away from the resin plate, peeled off at a certain speed, record the reading every 2cm, and calculate the average value.
[0050] 3.2 Comprehensive Appearance Score
[0051] Taking peelability, spreadability, paste uniformity, skin followability and shaping as the appearance evaluation indicators, they are scored separately (full score for each item is 10 points), the highest adhes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com