Multiple-trace element medicinal composition for injection, and use thereof
A technology of trace elements and compositions, applied in the field of medicine, can solve the problems of unfavorable children with autism, affecting glucose metabolism, unsuitable for patients with hyperchloremia and acidosis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 Preparation of small water injection preparations of various trace element pharmaceutical compositions
[0047] Prescription: Zinc Gluconate Hydrate (C 12 h 22 o 14 Zn) 1741.94mg (calculated as zinc gluconate anhydrate)
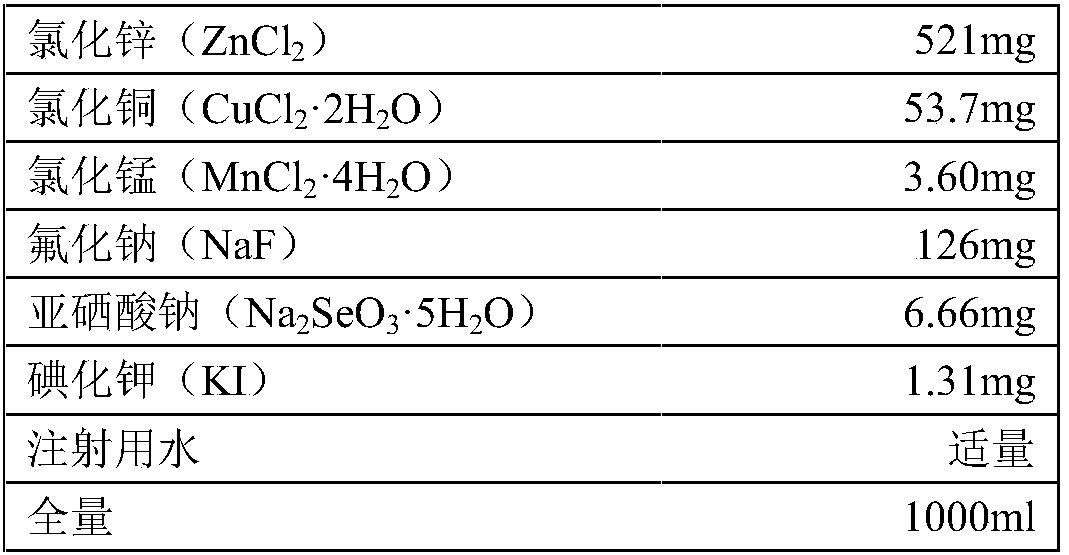
[0048] Copper chloride dihydrate (CuCl 2 2H 2 O) 53.7mg
[0049] Manganese chloride 4 hydrate (MnCl 2 4H 2 O) 3.60mg
[0050] Sodium fluoride (NaF) 126mg
[0051] Sodium selenite pentahydrate (Na 2 SeO 3 ·5H 2 O)6.66mg
[0052] Potassium iodide (KI) 1.31mg
[0053] Appropriate amount of 2M gluconic acid solution and 2M sodium gluconate solution
[0054] Appropriate amount of water for injection
[0055] Add water for injection to the full volume of 1000ml
[0056] Preparation process: Weigh the raw and auxiliary materials according to the prescription quantity, and use 90% of zinc gluconate hydrate, copper chloride 2 hydrate, manganese chloride 4 hydrate, sodium fluoride, sodium selenite 5 hydrate, and potassium iodide acco...
Embodiment 2
[0058] Example 2 Preparation of small water injection preparations of various trace element pharmaceutical compositions
[0059] Prescription: Zinc gluconate trihydrate 1741.94mg (weight based on zinc gluconate anhydrate), copper chloride dihydrate 53.7mg, manganese chloride 4hydrate 3.60mg, sodium fluoride 126mg, sodium selenite 5 Hydrate 6.66mg, sodium iodide 1.18mg, taurine 50mg, appropriate amount of 2M lactic acid solution and 2M sodium lactate solution, appropriate amount of water for injection, add water for injection to the full amount of 1000ml
[0060] Preparation process: Weigh the raw and auxiliary materials according to the prescription quantity, and use 85% of the prescription quantity of fresh Stir and dissolve the water for injection, adjust the pH value to 3.5 with 2M lactic acid solution and 2M sodium lactate solution, add 0.04% activated carbon (W / V) in the solution volume, stir and absorb for 15 minutes, and circulate decarbonization for 20 minutes; add wat...
Embodiment 3
[0061] Example 3 Preparation of Small Water Injection Preparation of Various Trace Element Pharmaceutical Compositions
[0062] Prescription: Zinc Gluconate (C 12 h 22 o 14 Zn) 1741.94mg, copper gluconate dihydrate 154.31mg, manganese gluconate dihydrate 8.754mg, sodium fluoride 126mg, sodium selenite pentahydrate 6.66mg, potassium iodide 1.31mg, 3M gluconic acid solution and 2M gluconic acid Appropriate amount of sodium aqueous solution, appropriate amount of water for injection, add water for injection to the full amount of 1000ml
[0063] Preparation process: Weigh the raw and auxiliary materials according to the prescription quantity, and use 92% of the prescription quantity accordingly to zinc gluconate, copper gluconate 2 hydrate, manganese gluconate 2 hydrate, sodium fluoride, sodium selenite 5 hydrate, and potassium iodide Stir and dissolve fresh water for injection, adjust the pH value to 4.2 with 3M gluconic acid solution and 2M sodium gluconate solution, add 0.02...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com