Method for recycling lithium battery copper current collector by using sodium sulfite
A technology using sodium sulfite and sodium sulfite, applied in battery recycling, recycling technology, recycling by waste collectors, etc., can solve the problems of complicated mechanical stripping process, difficulty in achieving uniformity, and low separation efficiency, and achieve simple process, low cost, Avoid complex effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
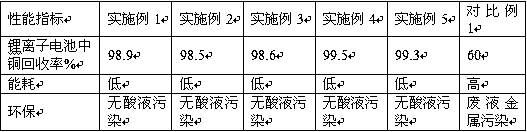
Examples
Embodiment 1
[0031] (1) Take out the cathode material of the lithium battery from the waste lithium battery, wash the cathode material with a mixed solution of acetone and ethanol at a volume ratio of 1:2, remove the organic solvent and organic binder, and then pulverize by a ball mill. Obtain the pretreated cathode material powder with a particle size of less than 300 mesh, the rotation speed of the ball mill is controlled at 50 pm, and the ball milling time is controlled at 2 hours;
[0032] (2) Mix the pretreated cathode material powder with 13% dilute hydrochloric acid, and keep stirring at a constant speed for 2 hours. After the cathode material is dissolved in the dilute hydrochloric acid, add excess sodium sulfite to form cuprous chloride-chlorine Sodium sulfide complex, let stand for 10 minutes, wait until precipitation is complete;
[0033] (3) Filter the mixed solution of the cuprous chloride-sodium chloride complex, take the clear solution and dilute it with the aqueous solution to o...
Embodiment 2
[0036] (1) Take out the lithium battery cathode material from the waste lithium battery, wash the cathode material with a 1:3 mixed solution of acetone and ethanol by volume to remove organic solvents and organic binders, and then pulverize with a ball mill. Obtain the pretreated cathode material powder with a particle size of less than 300 mesh, the rotation speed of the ball mill is controlled at 80 rpm, and the ball milling time is controlled at 2.5 hours;
[0037] (2) Mix the pretreated cathode material powder with 15% dilute hydrochloric acid, and keep stirring at a constant speed for 2.5 hours. After the cathode material is dissolved in the dilute hydrochloric acid, add excess sodium sulfite to form cuprous chloride-chlorine Sodium sulfide complex, let stand for 10 minutes, wait until precipitation is complete;
[0038] (3) Filter the mixed solution of the cuprous chloride-sodium chloride complex, take the clear solution and dilute it with the aqueous solution to obtain a cop...
Embodiment 3
[0041] (1) Take out the lithium battery cathode material from the waste lithium battery, wash the cathode material with a mixed solution of acetone and ethanol at a volume ratio of 1:4, remove organic solvents and organic binders, and then pulverize through a ball mill. Obtain the pretreated cathode material powder with a particle size of less than 300 mesh, the rotation speed of the ball mill is controlled at 160 rpm, and the ball milling time is controlled at 3.5 hours;
[0042] (2) Mix the pretreated cathode material powder with dilute hydrochloric acid with a concentration of 18%, and keep stirring at a constant speed for 3.5 hours. After the cathode material is dissolved in dilute hydrochloric acid, add excess sodium sulfite to form cuprous chloride-chlorine Sodium sulfide complex, let stand for 25 minutes, wait until precipitation is complete;
[0043] (3) Filter the mixed solution of the cuprous chloride-sodium chloride complex, take the clear solution and dilute it with the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com