A method for directional immobilization of lipase by nano-iron ferric oxide
A technique for immobilizing lipase and ferric oxide, which is applied to biochemical equipment and methods, and enzymes immobilized on or in inorganic carriers, can solve the problem of reduced enzyme activity, loss of immobilized enzyme activity, and poor performance. Improve enzyme performance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Nanometer Fe 3 o 4 Synthesis
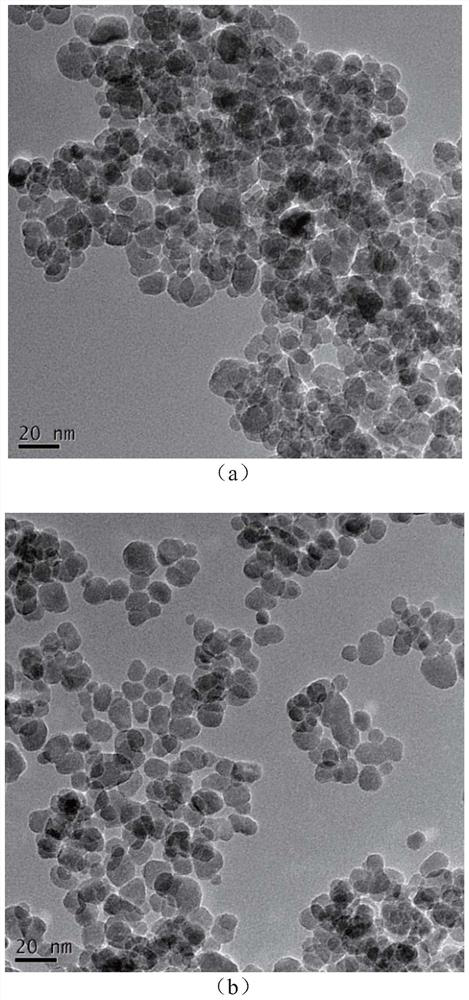
[0065] 11.75g FeCl 3 ·6H 2 O, 5.97g FeSO 4 ·7H 2 O was dissolved in 250ml of deionized water, heated to 80°C, and 14ml of ammonia water was quickly added under rapid stirring, and kept under nitrogen protection for 2h. The precipitate was washed with deionized water until the aqueous solution was neutral to obtain Fe 3 o 4 . Its transmission electron microscope as figure 1 (a) shown.
[0066] will get Fe 3 o 4 Add 500ml of 80% ethanol, slowly add 20ml of APTES (3-aminopropyl-triethoxysilane) at 30°C, react at room temperature for 12h, wash with deionized water and ethanol three times to obtain aminated Fe 3 o 4 . Aminated Fe 3 o 4 Add it to 100ml of 2% glutaraldehyde solution, react at 30°C for 2h, wash and freeze-dry to get about 5g of Fe with aldehyde groups on the surface 3 o 4 . Its transmission electron microscope as figure 1 (b) shown.
[0067] Depend on figure 1 (a) and figure 1 (b) It can be seen ...
Embodiment 11~18
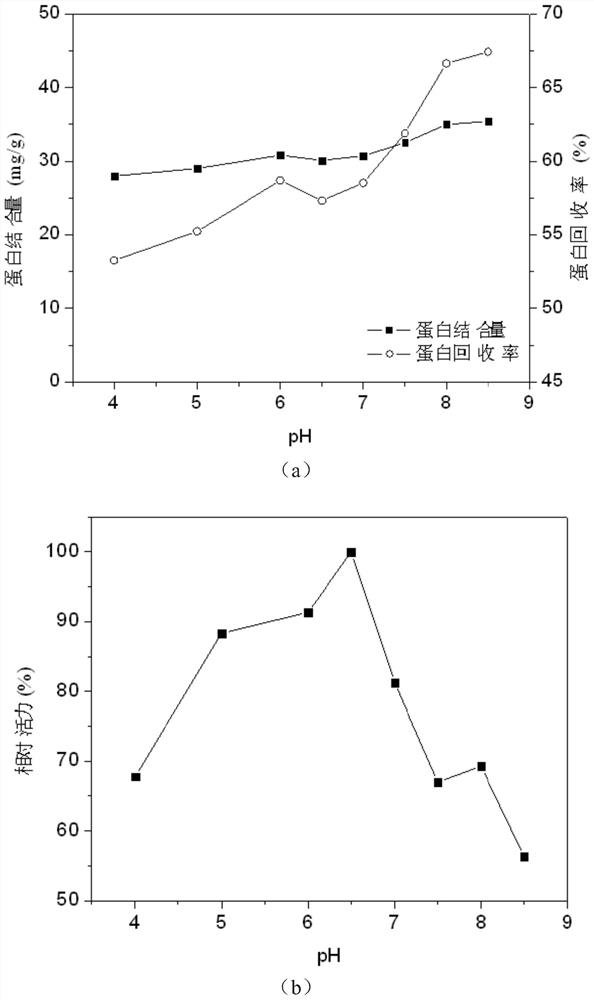
[0075] Effects of different pH values of Examples 11 to 18 on the activity of immobilized lipase
[0076] Get 1g Rhizopus oryzae lipase enzyme powder (protein content is 10.5%) and be dissolved in 25ml pH respectively in the phosphate buffer saline (100mM) of 4,5,6,6.5,7,7.5,8 and 8.5, add final concentration 1% (w / v) sucrose ester SE-11, stirred to dissolve the enzyme powder, then added 2 g of the activated ferric iron tetroxide prepared in Example 1, stirred and reacted at 20°C for 4 hours, and added a final concentration of 1 % (v / v) glutaraldehyde, continue to react for 2h. The obtained immobilized lipase is separated with a strong magnet, and washed with buffer solution for several times to wash away unbound protein and interfacial activator to obtain ferric oxide immobilized lipase.
[0077] Measure the relative activity of protein recovery rate, protein binding amount and ferric iron tetroxide immobilized lipase in each embodiment (defining the highest relative activ...
Embodiment 19~26
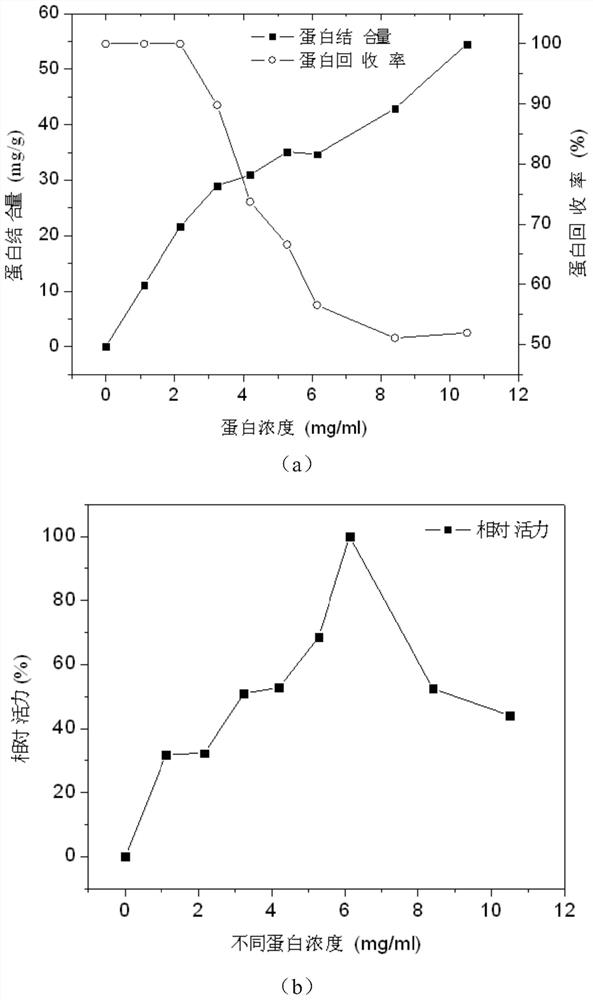
[0079] Examples 19-26 Effects of different enzyme protein concentrations on the activity of immobilized lipase
[0080] Get different quality Rhizopus oryzae lipase enzyme powder (protein content is 10.5%) and be dissolved in 25ml pH and be respectively in the phosphate buffer saline (100mM) of 6.5, make the protein concentration in the solution be respectively 1.1,2.2,3.2,4.2, 5.3, 6, 8.4, 10.5mg / ml, add the sucrose ester SE-11 that final concentration is 1% (w / v), stir to make enzyme powder dissolve, then add the activated iron ferric oxide prepared in 2g embodiment 1, After stirring and reacting at 20° C. for 4 h, glutaraldehyde with a final concentration of 1% (v / v) was added, and the reaction was continued for 2 h. The obtained immobilized lipase is separated with a strong magnet, and washed with buffer solution for several times to wash away unbound protein and interfacial activator to obtain ferric oxide immobilized lipase.
[0081] Determination of the relative activi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com