Method for synthesizing 1,2-bibenzyl compound
A technology for diarylethane and compounds, which is applied in the field of synthesizing 1,2-diarylethane compounds, can solve the problems of unfavorable large-scale industrial application, narrow substrate applicability, and high price, so as to avoid external compounding The use of body, good catalytic activity, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
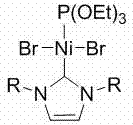
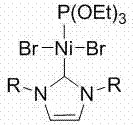
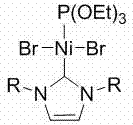
[0040] Example 1 Ni[P(OEt) 3 ][(RNCHCHNR)C]Br 2 Synthesis
[0041] Nitrogen heterocyclic carbene (RNCHCHNR) C (0.9470 g, 1.0 mmol) was added to a tetrahydrofuran solution of bis(triethylphosphite) nickel(II) bromide (0.5500 g, 1.0 mmol), and reacted at 60°C Overnight, the solvent was removed in vacuo, the residue was washed with n-hexane, the obtained residue was extracted with tetrahydrofuran, the clear liquid was transferred and the solvent tetrahydrofuran was removed to obtain a red solid as a divalent nickel (II) complex with a yield of 60%.
[0042] The product was subjected to elemental analysis, and the results are shown in Table 1:
[0043] Table 1 Elemental analysis results
[0044]
C:(%)
H:(%)
N:(%)
theoretical value
67.69
5.45
2.10
actual value
67.87
5.61
2.06
[0045] The product was characterized by NMR, and the results are as follows:
[0046] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal t...
Embodiment 2
[0051] Example 2 The divalent nickel (II) complex is used as a catalyst to catalyze the cross-coupling reaction of benzoheteroarenes and styrene compounds
[0052] Under argon protection, catalyst (66 mg, 0.05 mmol, 10 mol%), magnesium chips (12.0 mg, 0.5 mmol), benzothiazole (54 μl, 0.5 mmol), benzene Ethylene (86 microliters, 0.75 mmol), tetrahydrofuran (1.5 milliliters) were used as solvents, reacted at 80 °C for 60 hours, terminated the reaction with water, extracted the reaction product with ethyl acetate, and purified it by column chromatography (using ethyl acetate / petroleum A mixed solvent with a volume ratio of ether of 1:20 was used as a developer), and the yield was 90%.
[0053] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature:1 H NMR (400 MHz, CDCl 3 ) δ 7.99 (d, J = 8.1 Hz, 1H), 7.81 (d, J = 7.9 Hz,1H), 7.51 – 7.39 (m, 1H), 7.39 – 7.15 (m, 6H), 4.27 – 3.30 (...
Embodiment 3
[0054] Example 3 The divalent nickel (II) complex is used as a catalyst to catalyze the cross-coupling reaction of benzoheteroarenes and styrene compounds
[0055] Under the protection of argon, the catalyst (66 mg, 0.05 mmol, 10 mol%), magnesium chips (12.0 mg, 0.5 mmol), benzoxazole (51 μl, 0.5 mmol), and Styrene (86 microliters, 0.75 mmol), tetrahydrofuran (1.5 milliliters) were used as solvents, reacted at 90 °C for 36 hours, and terminated the reaction with water. The reaction product was extracted with ethyl acetate and purified by column chromatography (with ethyl acetate / A mixed solvent with a volume ratio of petroleum ether of 1:20 was used as a developer), and the yield was 89%.
[0056] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.63−7.55 (m, 1H), 7.43−7.34 (m, 1H), 7.26−7.09 (m, 7H), 3.14 (s, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com