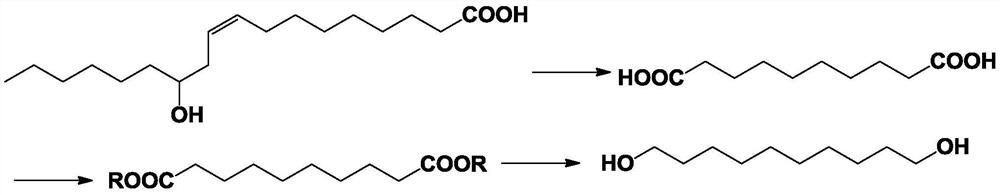
A kind of preparation method of 1,10-decanediol
A technology of decanediol and decenoic acid ester, which is applied in the field of preparation of 1,10-decanediol, can solve the problems of low total yield, low total product yield, difficult practical application value, etc., and increase the conversion rate From and selectivity, improving yield and selectivity, and solving the effect of product separation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]Add 184.15 g 9-decenoic acid methyl ester, 209.3 g tert-butanol solution containing 47.3 wt% tert-butyl hydroperoxide (TBHP) and 65.2 mg molybdenum acetylacetonate to the flask, replace the flask with nitrogen 3 times, Reaction at 60℃ for 2 hours, the conversion rate of 9-decenoic acid methyl ester is 99.8% by gas-phase internal standard method (qualitative and quantitative) and 1H NMR measurement (assisted qualitative), the choice of 9,10-epoxydecanoic acid methyl ester The purity is 98.6%. 191.1 grams of pure methyl 9,10-epoxydecanoate is obtained by distillation. Starting from methyl 9-decenoate, the yield of methyl 9,10-epoxydecanoate is 95.5% .
[0056]Add 70.0 g of 9,10-epoxydecanoic acid methyl ester and 1 ml of catalyst solution (615 mg of PdCl2Dissolve in 1000 ml of dichloromethane, take 1 ml of it), add 440.9 g of ammonium formate and 616 mg of vitamin C, replace the flask with nitrogen 3 times, react at 50°C for 300 minutes, measured by gas phase internal standard meth...
Embodiment 2
[0058]Add 200 grams of 9-decenoic acid methyl ester, 506.6 grams of ethylbenzene solution containing 35.5% by weight of ethylbenzene hydrogen peroxide (EBHP) and 1.84 grams of molybdenum naphthenate to the flask. Replace the flask with nitrogen 3 times at 200°C. After 3.5 hours of reaction, the conversion rate of 9-decenoic acid methyl ester was determined by gas phase internal standard method and 1HNMR to be 99.2%, the selectivity of 9,10-epoxydecanoic acid methyl ester was 98.3%, and 9,10 was obtained by distillation. -209.5 grams of pure methyl epoxydecanoate. Starting from methyl 9-decenoate, the yield of methyl 9,10-epoxydecanoate is 96.4%.
[0059]Add 90.0 g 9,10-epoxydecanoic acid methyl ester and 1 ml catalyst solution (5.034 g Pd(OAc) to the reaction flask2Dissolve in 1000ml of dichloromethane, take 1ml of it), add 1528.7g of sodium formate and 194mg of vitamin E, replace the flask with nitrogen 3 times, react at 80°C for 10 seconds, determine by gas phase internal standard me...
Embodiment 3
[0061]Add 120 grams of 9-decenoic acid methyl ester, 204 grams of cumene solution containing 55.8 wt% cumene hydroperoxide (CHP) and 0.39 grams of molybdenum stearate to the flask. Replace the flask with nitrogen 3 times. After reacting at 120°C for 12 hours, the conversion rate of 9-decenoic acid methyl ester was determined by gas-phase internal standard method and 1HNMR to be 99.5%, and the selectivity of 9,10-epoxydecanoic acid methyl ester was 99.1%. 9 , 124 grams of pure 10-epoxydecanoate methyl ester, starting from 9-decenoic acid methyl ester, the yield of 9,10-epoxydecanoic acid methyl ester is 95.1%.
[0062]Add 150.0 g of 9,10-epoxydecanoic acid methyl ester and 1 ml of catalyst solution (96.6 mg of CoCl2Dissolve in 1000 ml of dichloromethane, take 1 ml of it), then add 737.2 g of formic acid / triethylamine azeotrope (the content of formic acid is 46.8wt%, based on the total mass of formic acid and triethylamine azeotrope) And 2.7 g of glucose, replace the flask with nitrogen ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com