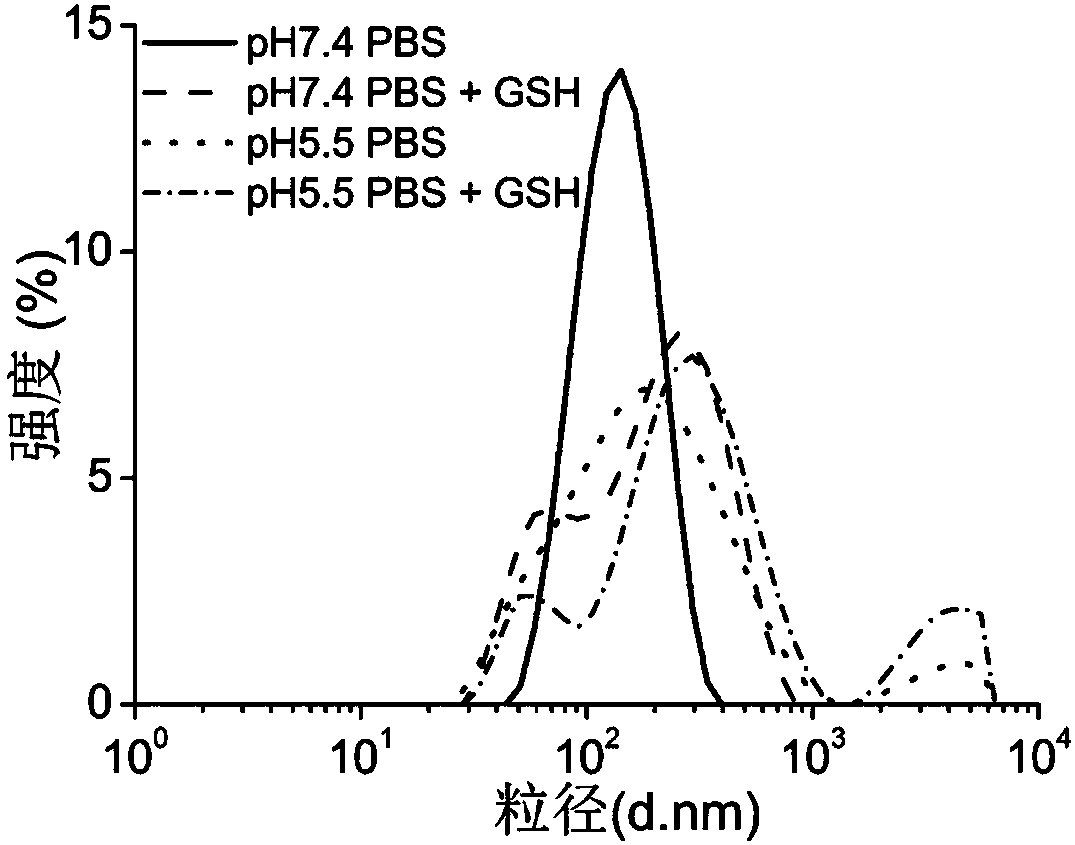
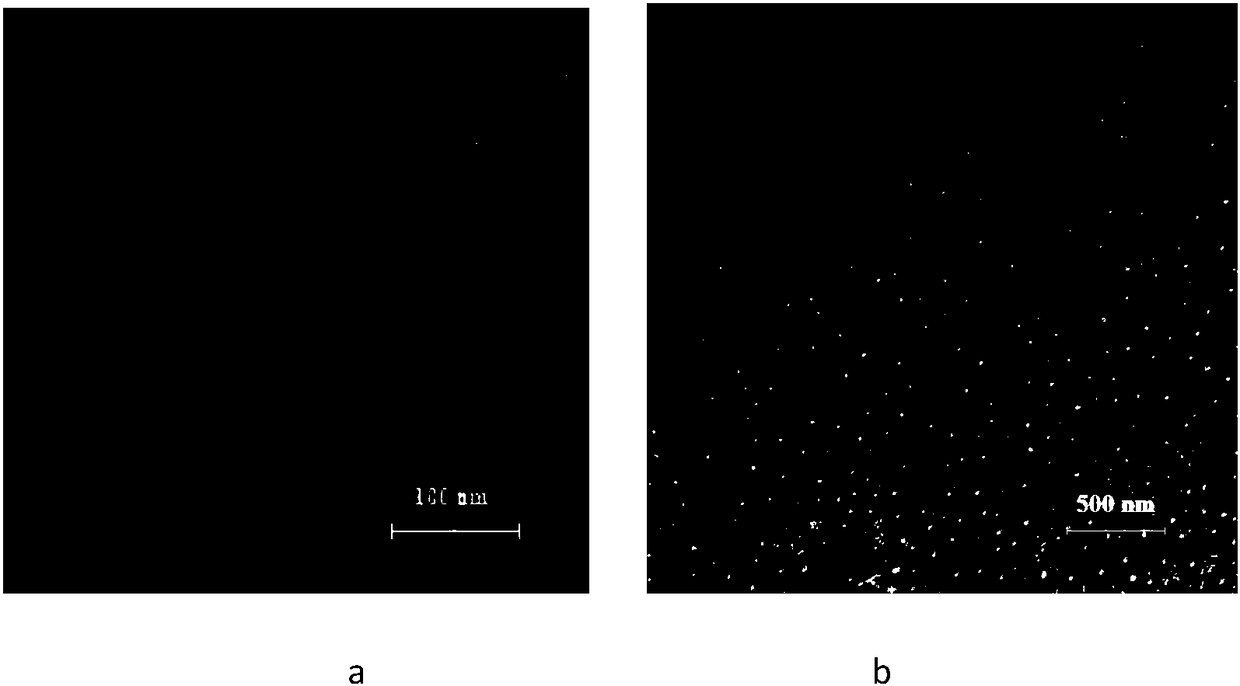
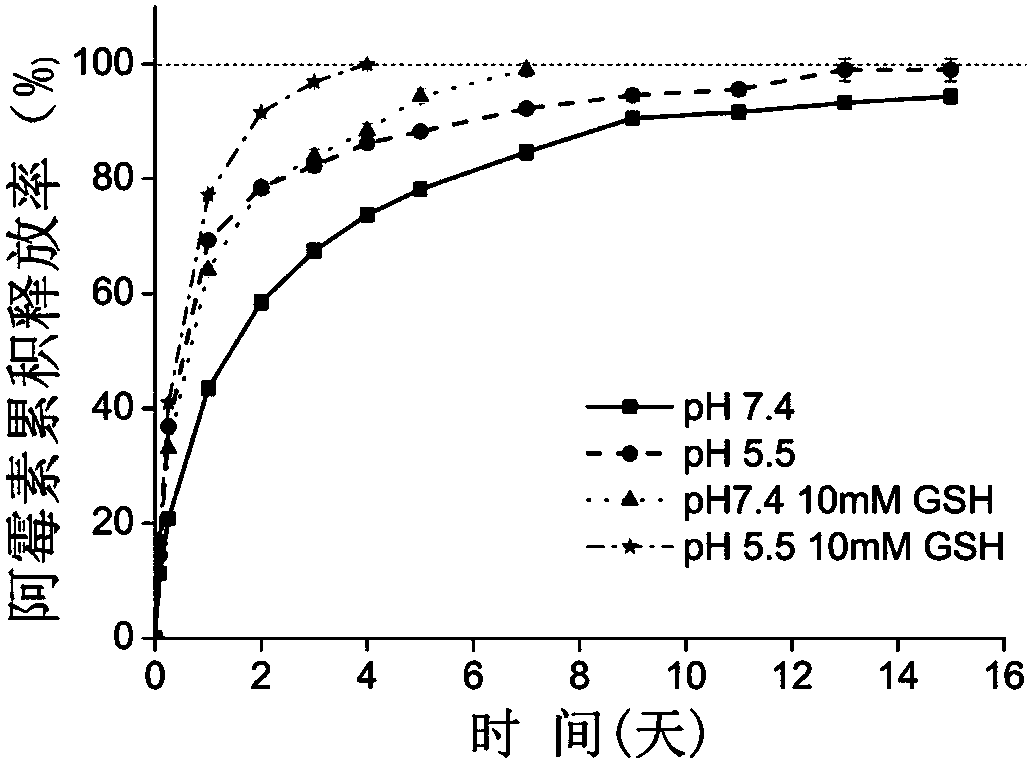
PH/reduction dual-sensitive multifunctional nano-micelle for tumor chemotherapy and photothermal combined therapy and application of pH/reduction dual-sensitive multifunctional nano-micelle
A nanomicelle, combined therapy technology, applied in antitumor drugs, drug combinations, medical preparations with non-active ingredients, etc., can solve the problems of tumor cell apoptosis, ablation, necrosis, etc., and achieve enhanced uptake and accelerated drug release. , the effect of high drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Both PCL-ss-PEG-ss-PCL and PCL-acetal-PEG were self-synthesized by the research group (for the synthesis method of PCL-ss-PEG-ss-PCL, please refer to the applicant’s patent application on the same day, patent name: folic acid targeted reduction sensitive carrier Drug polymer nanomicelle and its preparation method and application; PCL-acetal-PEG synthesis method, see the applicant's patent application on the same day, patent name: semi-solid acid-sensitive amphiphilic block copolymer preparation method and its use). PCL-ss-PEG-ss-PCL is polycaprolactone-disulfide bond-polyethylene glycol-disulfide bond-polycaprolactone, molecular weight This example uses PCL 3750 -ss-PEG 7500 -ss-PCL 3750 , but when the molecular weight of the PCL-ss-PEG-ss-PCL copolymer is 12000-16000, it can be prepared when the mass percentage of the PEG hydrophilic segment is >45%. PCL-acetal-PEG is polycaprolactone-acetal-polyethylene glycol, and the molecular weight used in this embodiment is 180...
Embodiment 2
[0060] A method for preparing pH / reduction double-sensitive drug-loaded polymer micelles (co-delivery polymeric micelles, Co-PMs), comprising the following steps:
[0061] (1) First transfer doxorubicin hydrochloride and ICG to hydrophobicity respectively: add 1% (mass percentage of material) doxorubicin hydrochloride to methanol solution containing triethylamine (20 μL triethylamine / 1mg doxorubicin), React at room temperature for 20 hours under magnetic stirring; mix ICG and tetrabutylammonium iodide (1:5.72 mass ratio) in dichloromethane, and react at room temperature for 20 hours under magnetic stirring.
[0062] (2) Fully dissolve PCL-ss-PEG-ss-PCL and PCL-acetal-PEG amphiphilic copolymer 1:1 (mass ratio) in the organic solvent dichloromethane, then add desalted doxorubicin and transfection Mix the hydrophobic ICG solution evenly, remove the organic solvent with a rotary evaporator to form a uniform film on the inner wall of the eggplant-shaped bottle, blow dry the residua...
Embodiment 3
[0075] A method for the preparation of folate-targeted, pH / reduction dual-sensitive drug-loaded polymer micelles (FA Co-PMs). Including the following steps:
[0076] When preparing polymer micelles with folic acid-targeted double drug loading, 0.5%-5% (mass percentage) DSPE-PEG (2000)-NH 2 . According to the same steps as in Example 2 (1), (2), (3) and (4) to prepare the surface with -NH 2 After the drug-loaded polymer micelles of active groups, the folic acid targeting molecules were linked by EDC / NHS reaction. The specific reaction steps are: (1) Weigh the molar ratio of about DSPE-PEG-NH 2 5 times of folic acid, dissolved in 500 μL deionized water. (2) Add EDC with a molar ratio of about 10 times of folic acid and NHS with a molar ratio of 2 times of EDC to activate for 15 min. (3) Add the above-mentioned aminated nanoparticles and react at room temperature for 8 hours under magnetic stirring. The specific reaction molecular structure formula is as follows, the carbox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com