Red fluorescent material and preparation method thereof
A technology of red fluorescent and red fluorescent powder, applied in the field of materials science, can solve the problems of large environmental pollution and poor chemical stability of fluoride fluorescent powder, and achieve the effect of low cost, low cost and reducing the center of mass of excited states
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
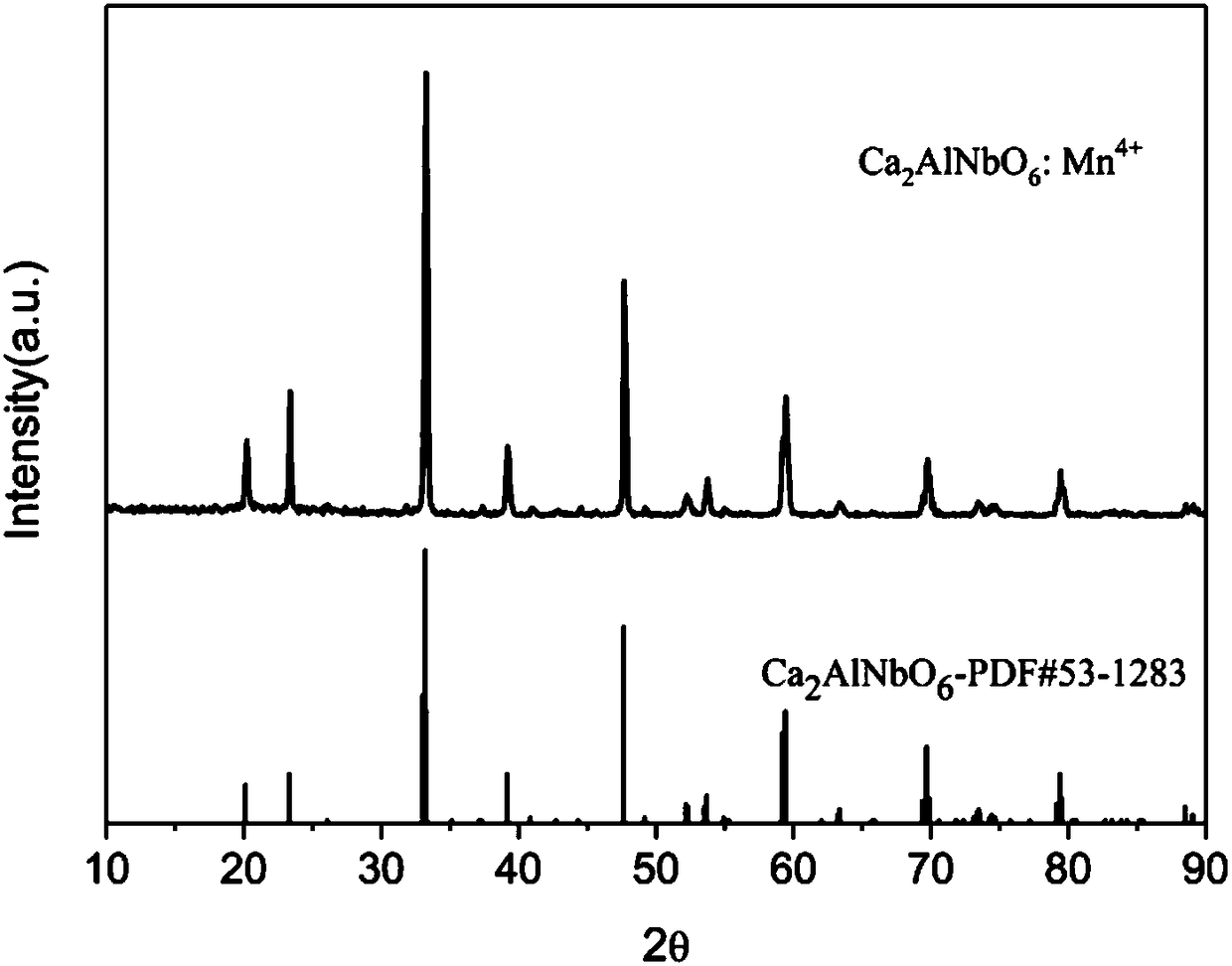
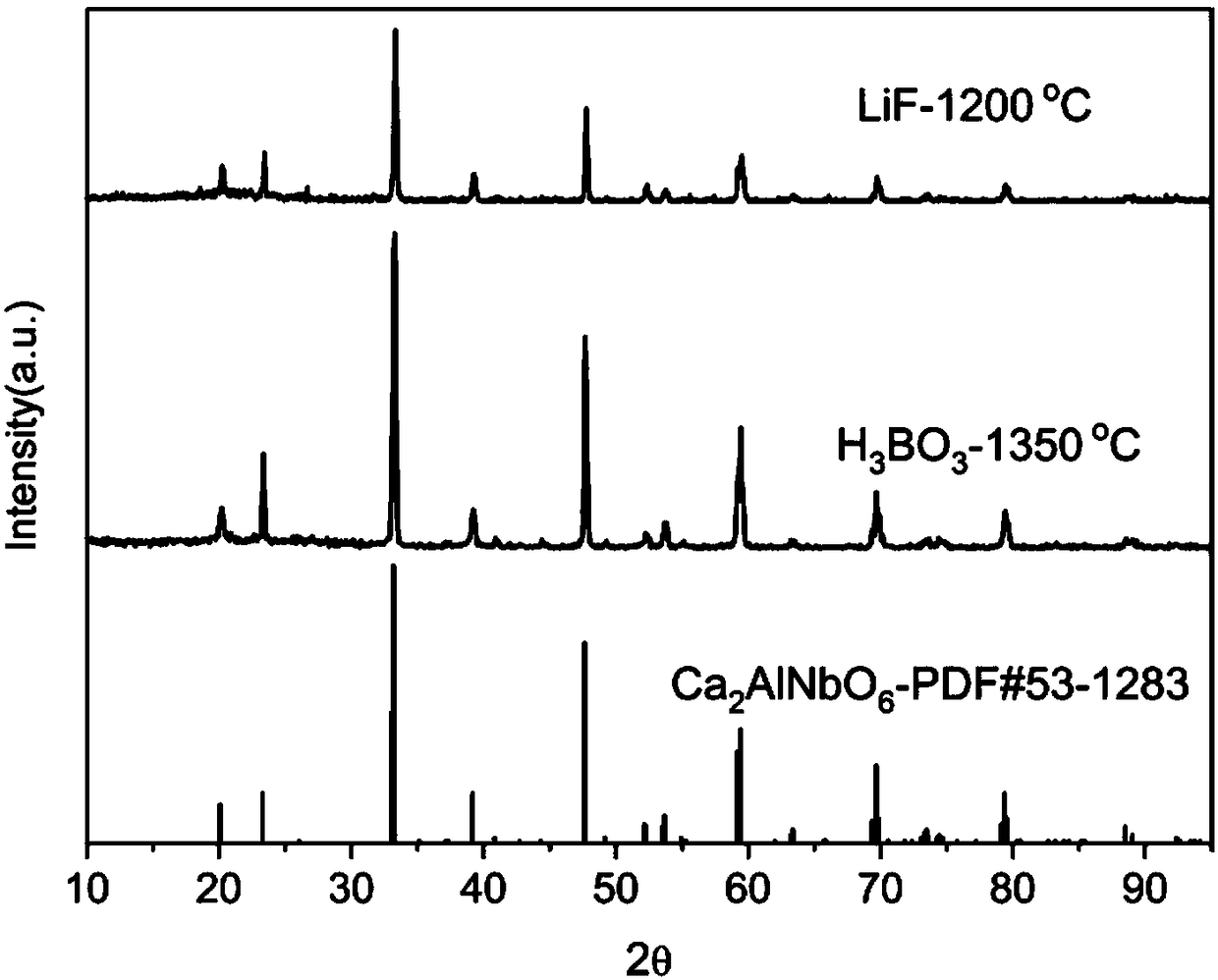
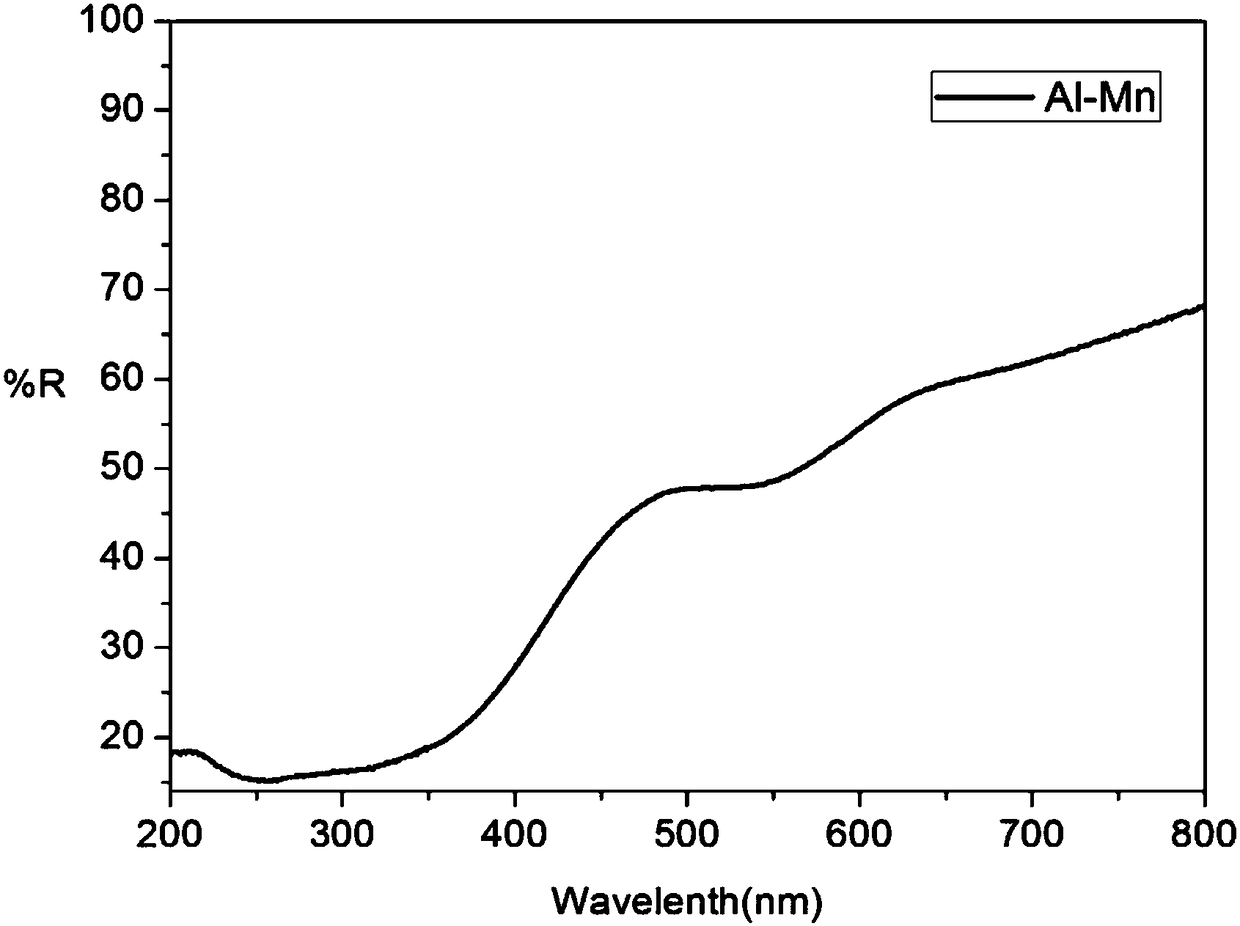
[0033] Raw material CaCO3 , Nb 2 o 5 , Al 2 o 3 , MnO 2 According to Ca 2 AlNbO 6 :xMn 4+ (x=0.02) stoichiometric ratio was weighed, mixed evenly in a mortar, put into a corundum crucible with a cover and pre-fired at 850°C for 12h, after taking it out, it was fully ground, and then placed at 1250°C, 1500°C and fired for 12h respectively to obtain Ca 2 AlNbO 6 :Mn 4+ Phosphor powder: crush the phosphor powder, remove impurities, wash with deionized water until neutral, dry and classify to obtain the finished product. The product is Ca 2 AlNbO 6 :Mn 4+ ,See figure 1 . The sample has an absorption band in the ultraviolet region and green region, respectively located at 355nm and 527nm. Among them, the excitation band in the ultraviolet region is the strongest (355nm), see image 3 . When the detection wavelength is 712nm, Ca 2 AlNbO 6 :Mn 4+ The excitation spectrum see Figure 4 . Under the excitation of short-wave ultraviolet (355nm), it shows red broadband...
Embodiment 2
[0035] Raw material CaCO 3 , Nb 2 o 5 , Al 2 o 3 , MnO 2 , MgO according to Ca 2 AlNbO 6 :xMn 4+ ,yMg 2+ (x = 0.01, y = 0.01) stoichiometric ratio was weighed, mixed evenly in a mortar, put into a corundum crucible with a cover and pre-fired at 950 ° C for 12 hours, after taking it out, it was fully ground, and then placed at 1300 ℃, fired at 1500℃ for 12h and 24h respectively to obtain Ca 2 AlNbO 6 :Mn 4 + ,Mg 2+ Powder, the fluorescent powder is crushed and removed, washed with deionized water until neutral, dried and classified to obtain the finished product. The sample has an absorption band in the ultraviolet region and a green region, respectively located at 355nm and 527nm, and the excitation band in the ultraviolet region is the strongest (355nm). Under short-wave ultraviolet (355nm) excitation, it shows a red broadband emission (620nm-800nm), with the strongest peak at 712nm.
Embodiment 3
[0037] Raw material CaCO 3 , Nb 2 o 5 , Al 2 o 3 , MnO 2 , CaO according to Ca 2 AlNbO 6 :xMn 4+ ,y Ca 2+ (x = 0.01, y = 0.01) stoichiometric ratio was weighed, mixed evenly in a mortar, put into a corundum crucible with a cover and pre-fired at 950 ° C for 12 hours, after taking it out, it was fully ground, and then placed at 1300 ℃, fired at 1500℃ for 12h and 24h respectively to obtain Ca 2 AlNbO 6 :Mn 4 + , Ca 2+ Powder, the fluorescent powder is crushed and removed, washed with deionized water until neutral, dried and classified to obtain the finished product. The sample has an absorption band in the ultraviolet region and a green region, respectively located at 355nm and 527nm, and the excitation band in the ultraviolet region is the strongest (355nm). Under short-wave ultraviolet (355nm) excitation, it shows a red broadband emission (620nm-800nm), with the strongest peak at 712nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com