Whitening liquid crystal composition as well as preparation method and application thereof
A liquid crystal composition and whitening technology, which can be applied in pharmaceutical formulations, cosmetic preparations, dressing preparations, etc., can solve the problems of insufficient whitening effect, single effect, skin irritation, etc., and achieve effective penetration promotion, good stability, and improved The effect of load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
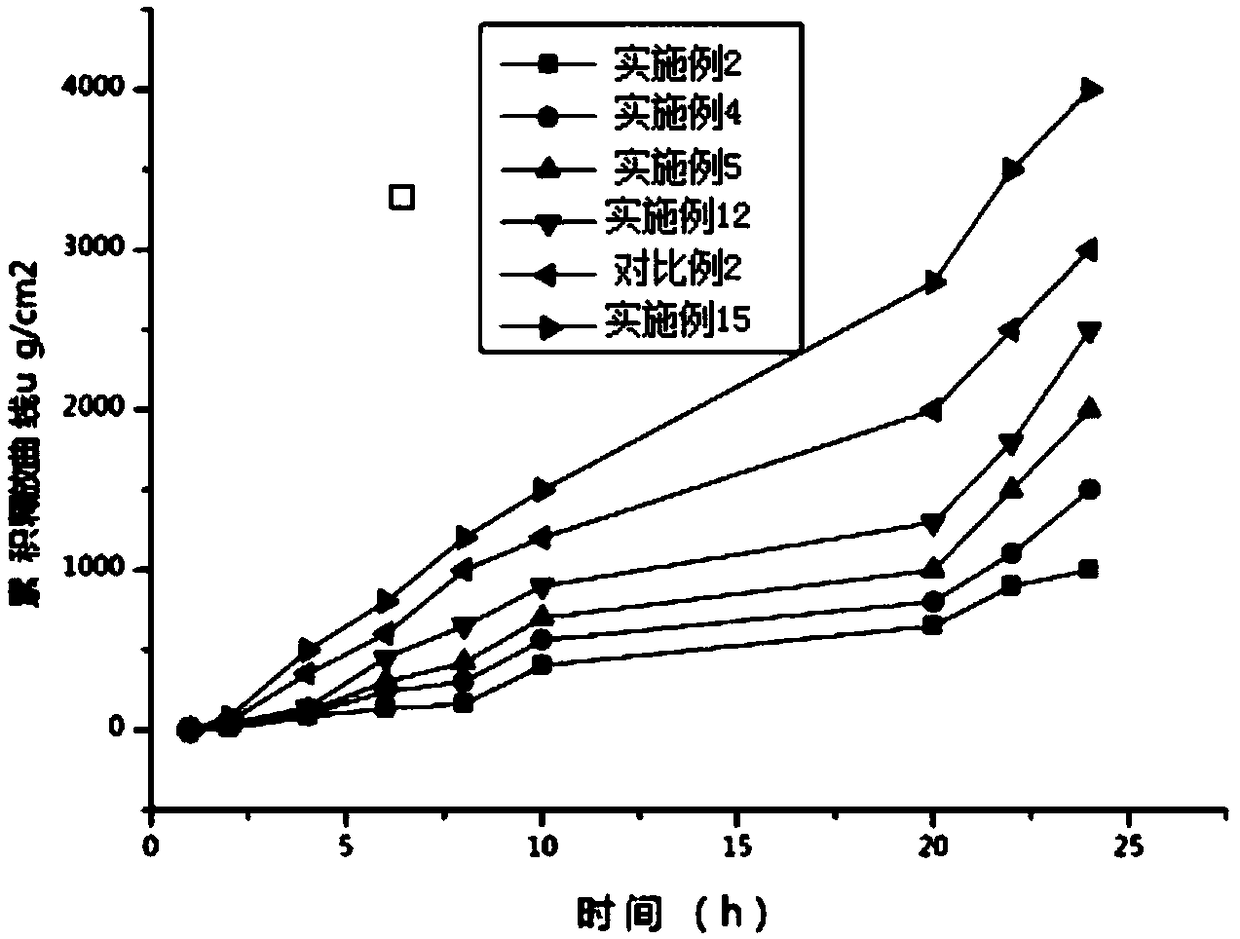
Image
Examples
preparation example Construction
[0083] The present invention also provides a preparation method of the whitening liquid crystal composition described in the above technical solution, comprising the following steps:
[0084] A. Dissolving liquid crystal emulsifier and non-water-soluble whitening active ingredient in liquid lipid to obtain oil phase;
[0085] B. Dissolving co-emulsifier and emulsification thickener in water to obtain water phase;
[0086] C. Dissolving the water-soluble whitening active ingredient in water to obtain an aqueous solution of the whitening active ingredient;
[0087] D. Mix and emulsify the oil phase obtained in step A with the water phase obtained in step B, and micronize it to obtain a micron-scale dispersion;
[0088] E. Mix and emulsify the aqueous solution of whitening active ingredients obtained in step C with the micron-sized dispersion obtained in step D, and perform nano-processing to obtain a whitening liquid crystal composition;
[0089] There is no sequence limitatio...
Embodiment 1
[0107] In terms of mass percentage, 1.0% soybean lecithin, 2.0% cetearyl alcohol (and) cocoyl glucoside, 4.0% isopropyl palmitate, 2.0% isopropyl myristate and 2% glabridin Melt in a water bath at 70°C to obtain an oil phase for later use;
[0108] Mix 0.1% ferulic acid, 0.1% arbutin, 1.0% nicotinamide, 0.1% VC and its derivatives, 1.0% glycyrrhizinic acid, 5.0% tea polyphenols and 30% water, under the condition of 35 ℃ water bath Stir to obtain the whitening active ingredient aqueous solution, and set aside;
[0109] Dissolve 2.0% polyethylene glycol, 3.0% hexanediol and 0.01% hydroxyethyl acrylate into 46.6% water, stir in a water bath at 70°C to obtain a water phase, and set aside;
[0110] Mix the water phase with the oil phase, and after the mixing is completed, high-speed shear emulsification for 5 minutes at a rotation speed of 5500 rpm to obtain a micron-sized dispersion;
[0111] The micron-scale dispersion is mixed with the whitening active ingredient aqueous solut...
Embodiment 2
[0113] In terms of mass percentage, 0.5% hydrogenated lecithin, 0.5% hydroxystearyl alcohol and hydroxystearoside, 2.0% caprylic capric triglyceride, 2.0% propylene glycol dioctylcaprate and 1.0% VE were placed in a water bath at 65°C Melt down to get the oil phase, set aside;
[0114] Mix 3.0% α-arbutin, 3.0% nicotinamide, 1.0% vitamin C ethyl ether, 2.0% silymarin with 35% water, stir in a water bath at 40°C to obtain an aqueous solution of whitening active ingredients, and set aside;
[0115] 2.0% stearyl alcohol, 3.0% hexanediol, 2.0% sodium acrylate / sodium acryloyldimethyl taurate copolymer (and) isohexadecane (and) polysorbate-80 and 3.0% Dissolve the bohm in 35% water, stir in a water bath at 65°C to obtain the water phase, and set aside;
[0116] Mix the water phase and the oil phase, and after the mixing is completed, high-speed shear emulsification for 1 min at a rotation speed of 5000 rpm to obtain a micron-sized dispersion;
[0117] Mix the micron-scale dispersio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com