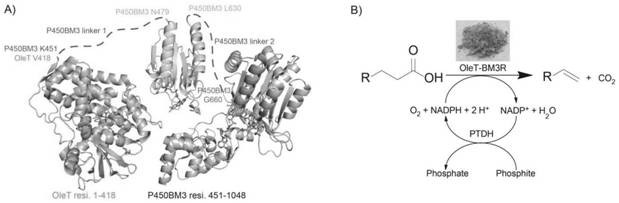
Sequence, preparation method and application of catalytic synthesis of linear alpha-olefin biocatalyst olet-bm3r
A biocatalyst, olet-bm3r technology, applied in the field of protein engineering, can solve the problems of severe reaction system conditions, low yield of enzyme reaction system, complex reaction system, etc., and achieve the effects of simplified reaction system, low energy consumption, and reduced production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The construction of the gene expression vector of embodiment 1 OleT-BM3R
[0031] The OleT-BM3R fragment (sequence shown in SEQ ID NO: 2, synthesized by Suzhou Jinweizhi Biological Co., Ltd.) synthesized by the whole gene was subjected to double enzymes by restriction enzymes NdeI and XhoI (New England Biolabs) according to the instructions. After cutting, T4 ligase (New England Biolabs) was used to connect to the expression vector pET28a that had been digested by NdeI and XhoI. The ligation product was used to transform Escherichia coli DH5α competent cells (Tiangen Biochemical Technology Co., Ltd.). Pick the successfully transformed monoclonal colony from the solid LB medium plate containing 50 μg / ml kanamycin, in the LB liquid medium containing the same concentration of kanamycin, at a temperature of 37°C, and the shaker speed is 220rpm cultured overnight under conditions. The recombinant plasmid was extracted from the cultured bacterial liquid with a small plasmid...
Embodiment 2
[0032] Example 2 Preparation of OleT-BM3R
[0033] (1) Transform the recombinant plasmid successfully sequenced into Escherichia coli Rosetta (DE3) (Tiangen Biochemical Technology Co., Ltd.), pick the positive clone into 5ml LB liquid containing 50μg / ml kanamycin and 34μg / ml chloramphenicol After culturing overnight in the medium, add the bacterial solution to 500ml TB liquid medium containing 50μg / ml kanamycin and 34μg / ml chloramphenicol at a ratio of 1:100, and carry out expansion cultivation at 37°C with a shaker speed of 220rpm . Waiting for OD 600 At about 0.6, the heme precursor δ-aminolevulinic acid and 0.1 mM IPTG were added at a final concentration of 0.5 mM for induction. After expressing for 20 hours at 22°C with a shaker speed of 220 rpm, the bacteria were harvested by centrifugation at a speed of 5000 rpm for 10 minutes. After discarding the supernatant medium, resuspend the bacteria with 50ml of buffer A (0.1M KPi potassium phosphate, 0.3MKCl, pH7.0), lyse the...
Embodiment 3
[0035] Example 3 Using OleT-BM3R freeze-dried powder combined with PTDH-based NADPH regeneration system to catalyze the synthesis of corresponding alpha-olefins from natural saturated fatty acids
[0036] Reaction system: buffer A, OleT-BM3R (3 μ M), fatty acid (1 mM) (fatty acid C4-C11 co-solvent: 5% EtOH (v / v), fatty acid C12-C20 co-solvent: 5% EtOH (v / v) and 1.5% Triton X-100 (v / v)), catalase (100U mL -1 ), sodium phosphite (10mM), PTDH phosphite dehydrogenase (2μM), NADPH (200μM), room temperature, shaker speed 125rpm, react for 12 hours. Yield of olefins synthesized by decarboxylation of natural fatty acids: C4: 14%, C5: 11%, C6: 68%, C7: 70%, C8: 40%, C9: 47%, C10: 70%, C11: 58%, C12: 46%, C14: 52%, C16: 60%, C18: 73%, C20: 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com