Novel carbazole fluorescent thiol marking reagent as well as synthesis method and application thereof
A technology for labeling reagents and synthesis methods, which is applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of high instrument cost, and achieve the effects of high labeling yield, short labeling time, and mild labeling conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
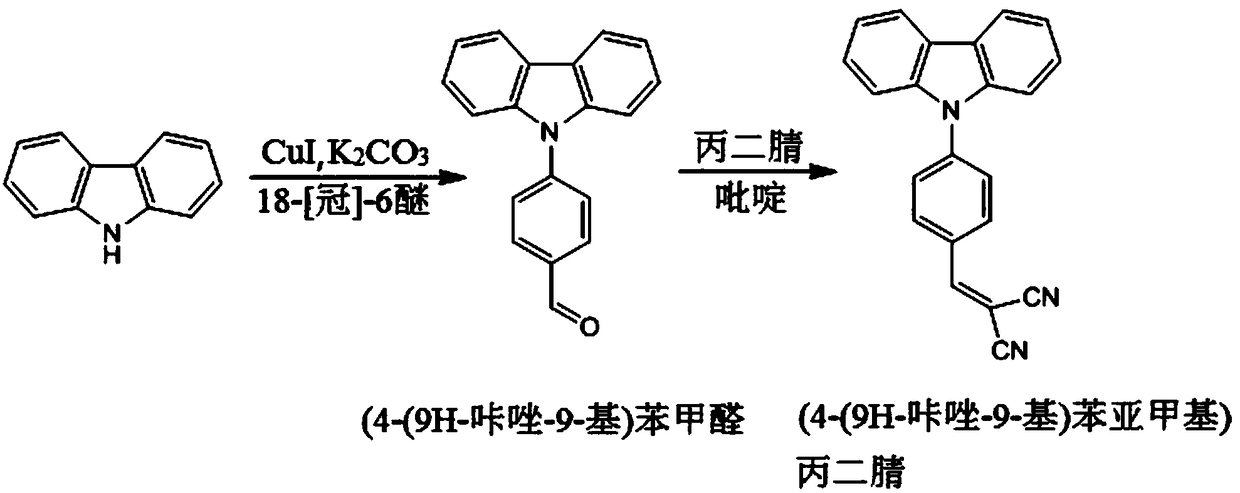
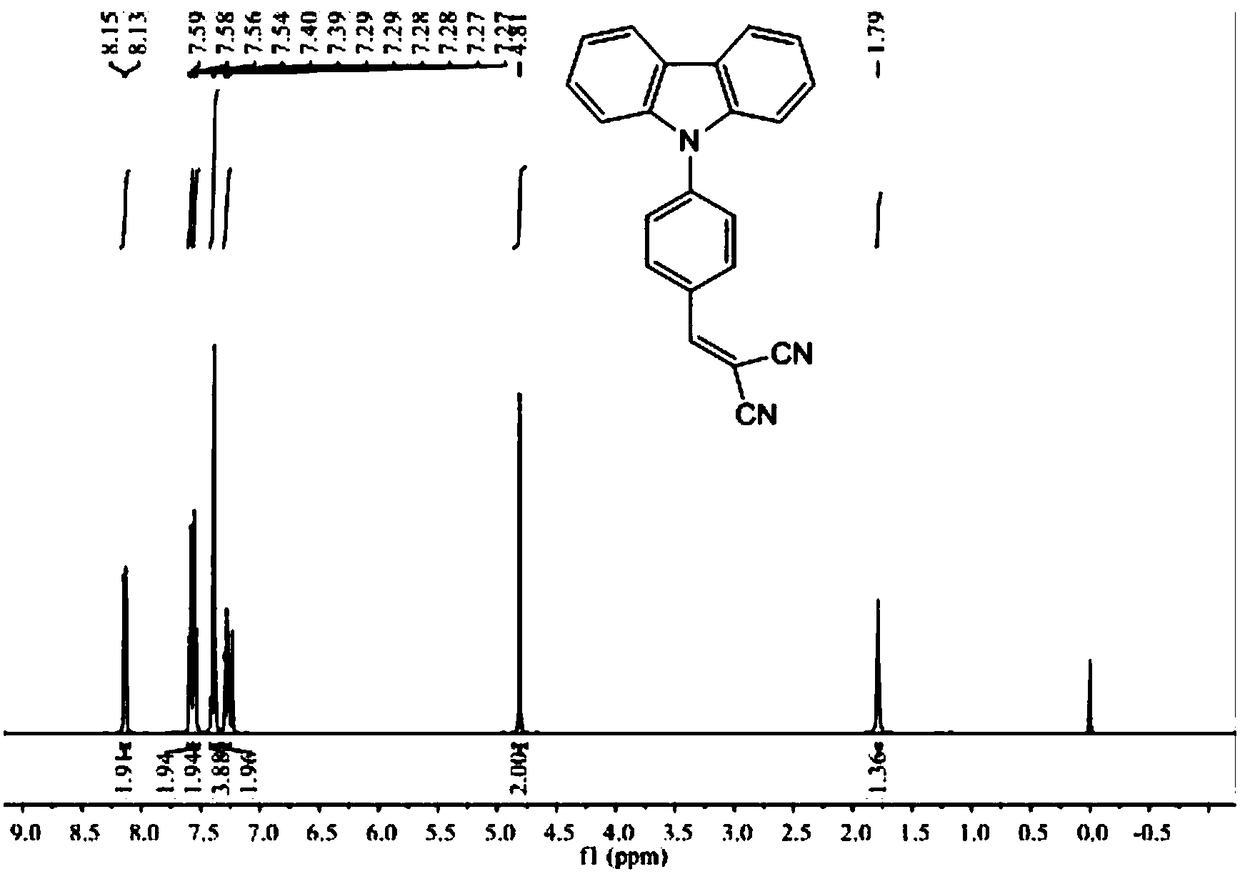
[0029] Preparation of (4-(9H-carbazol-9-yl)benzylidene)malononitrile
[0030] (1) Add 10g carbazole, 13.2g 4-bromobenzaldehyde, 5g potassium carbonate, 2.5g cuprous iodide and 2g18-[crown]-6 ether in a 250mL three-necked flask, add 150mL dimethyl sulfoxide as a solvent, Oil bath 130 ℃ reacted 24h; After the reaction was finished, the reaction solution was cooled to room temperature, suction filtered, then the suction filtrate was mixed with 20wt% NaCl aqueous solution to precipitate a solid, the solid was recovered and dried, and recrystallized 3 times with acetonitrile to obtain the intermediate ( 4-(9H-carbazol-9-yl)benzaldehyde, the yield is 85%;
[0031] (2) Add 7.5g (4-(9H-carbazol-9-yl) benzaldehyde to a 100ml three-necked flask equipped with electromagnetic stirring, completely dissolve with 20mL of anhydrous acetonitrile, 2mL of anhydrous pyridine as a catalyst, and then add 2.8 g malononitrile, heating and reflux reaction 4h, after the reaction finished, the reaction...
Embodiment 2
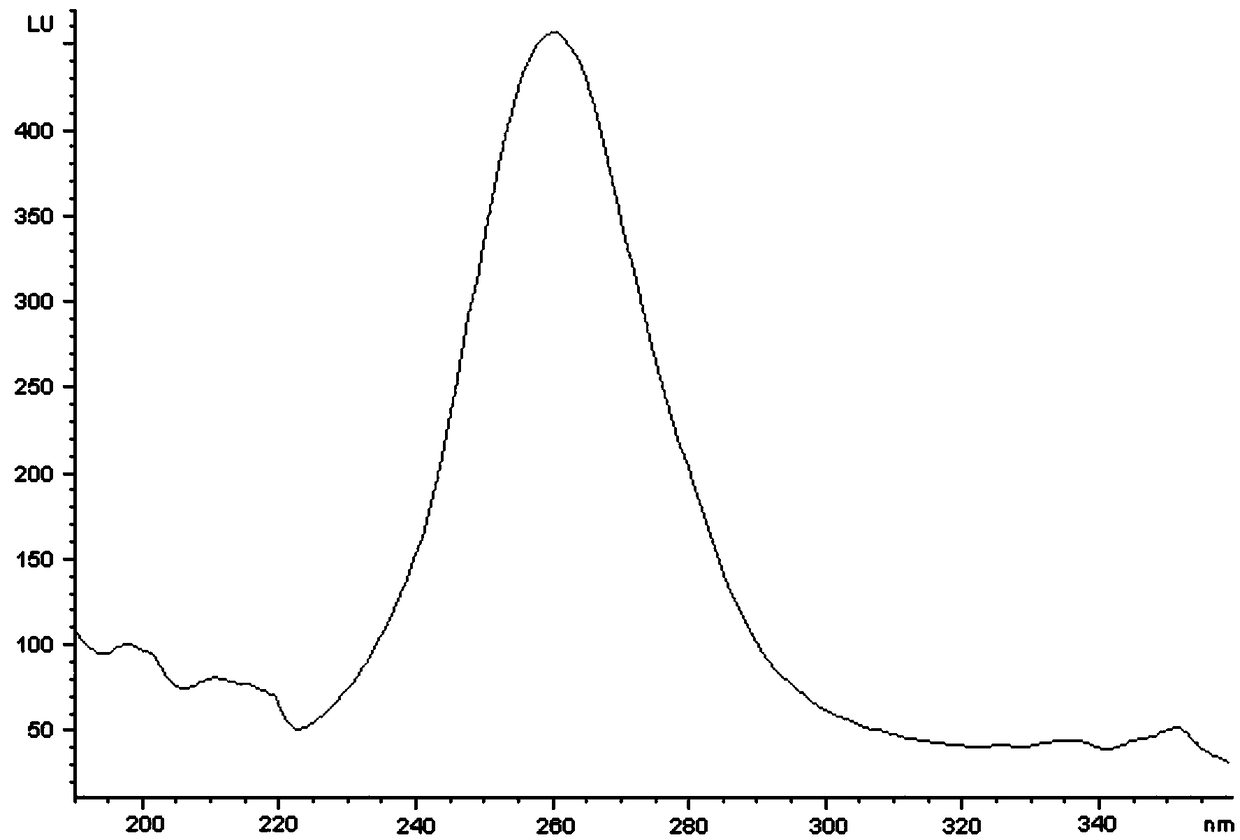
[0036] The thiol degradation products of organophosphorus thiol ester insecticides are marked by fluorescent thiol labeling reagents, so as to realize the quantitative analysis of organophosphorus thiol ester insecticides, except for the complete detection of organophosphorus thiol ester insecticides. In addition to hydrolysis and complete labeling of degraded thiol products, accurate acquisition of the fluorescence maximum excitation and maximum emission wavelengths of labeling reagents and degraded thiol product markers is the key to improving detection sensitivity and reducing detection limits.
[0037] The invention utilizes the nucleophilic substitution reaction between phenylbutane-1,2,3-triketone-2-oxime and organophosphorus thiol ester insecticides to release and degrade the hydrolyzed organophosphorus thiol ester insecticides Mercaptan products, by optimizing the amount of phenylbutane-1,2,3-trione-2-oxime and organophosphorus thiol ester insecticides, the complete pro...
Embodiment 3
[0040] (1) Utilize (4-(9H-carbazol-9-yl)benzylidene)malononitrile to fluorescently label the degraded thiol products of organophosphorus thiol ester pesticides to realize the organophosphorus thiol esters in agricultural products For the quantitative analysis of pesticides, prepare 2.0nM phenylbutane-1,2,3-trione-2-oxime solution and use it to catalyze the hydrolysis of organophosphorus thiol ester pesticides to produce degraded thiol products. Prepare 5 parts of solutions of organophosphorus thiol ester insecticide standards, the concentrations are respectively 1 μM, 10 μM, 100 μM, 1 mM, 10 mM; The acetonitrile solution of methyl) malononitrile, the 4-dimethylaminopyridine solution of preparation 3.0nM, provides suitable alkaline environment for the labeling reaction of labeling reagent and the thiol degradation product of organophosphorus thiol ester insecticide;
[0041] (2) Add 100 μL of the standard solution of organophosphorus thiol ester insecticides and 200 μL of pheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com