Iridium complex taking thiobis diaryl/aromatic heterophosphamide compound as auxiliary ligand
A technology of iridium complexes and ligands, applied to compounds containing elements of group 8/9/10/18 of the periodic table, indium organic compounds, platinum group organic compounds, etc., can solve carrier imbalance and reduce device Efficiency and other issues, to achieve the effect of simple preparation method, improved electron mobility, and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of Auxiliary Ligand Thiobisaryl / Aryl Heterophosphorimide
[0034] React bromobenzene and magnesium powder to obtain Grignard reagent, then react with phosphorus trichloride, diphenyl diphenylphosphorus chloride derivatives are distilled off under reduced pressure, and further react with hexamethylsilyl amine, and separate by column chromatography, Then oxidize with sulfur powder in tetrahydrofuran under anaerobic conditions to obtain thiobisdiphenylphosphorimide
[0035] Other thiobis-diaryl / aryl-heterophosphorimides can be prepared by the above method:
[0036]
Embodiment 2
[0037] The preparation of embodiment 2 iridium complexes of the present invention
[0038] The main ligand 2-phenylpyridine and IrCl 3 Reflux in the ethoxyethanol solution in the ratio of 2:1 for 10 hours, cool and filter to obtain the chlorine bridge complex of iridium; The potassium hydroxide of base phosphoramidite auxiliary ligand and 20mmol (1.12 grams) was refluxed in ethoxyethanol for two hours to obtain the crude product of iridium complex, and column chromatography obtains 17.28 grams of pure products Stpip 1 (productivity: 91%)
[0039] Further place 5 g of Stpip 1 in the quartz tube at 10 -5 Heating and sublimation purification under Pa vacuum conditions obtained 4.5 grams of luminescent material (sublimation rate 90%) that met the requirements for device preparation. The response looks like this:
[0040]
[0041] Gained iridium complex Stpip 1 is analyzed as follows by proton nuclear magnetic resonance spectrum and high resolution mass spectrometry:
[004...
Embodiment 3
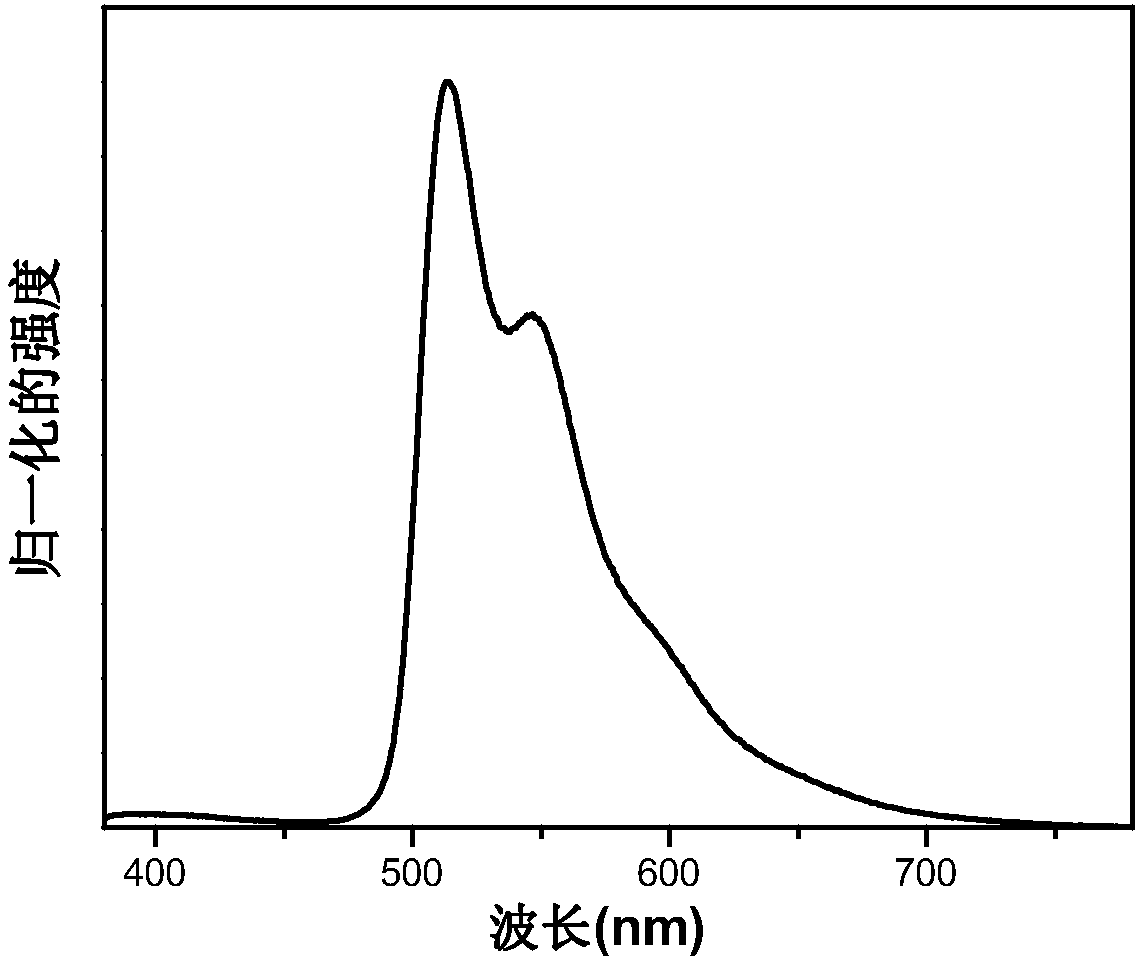
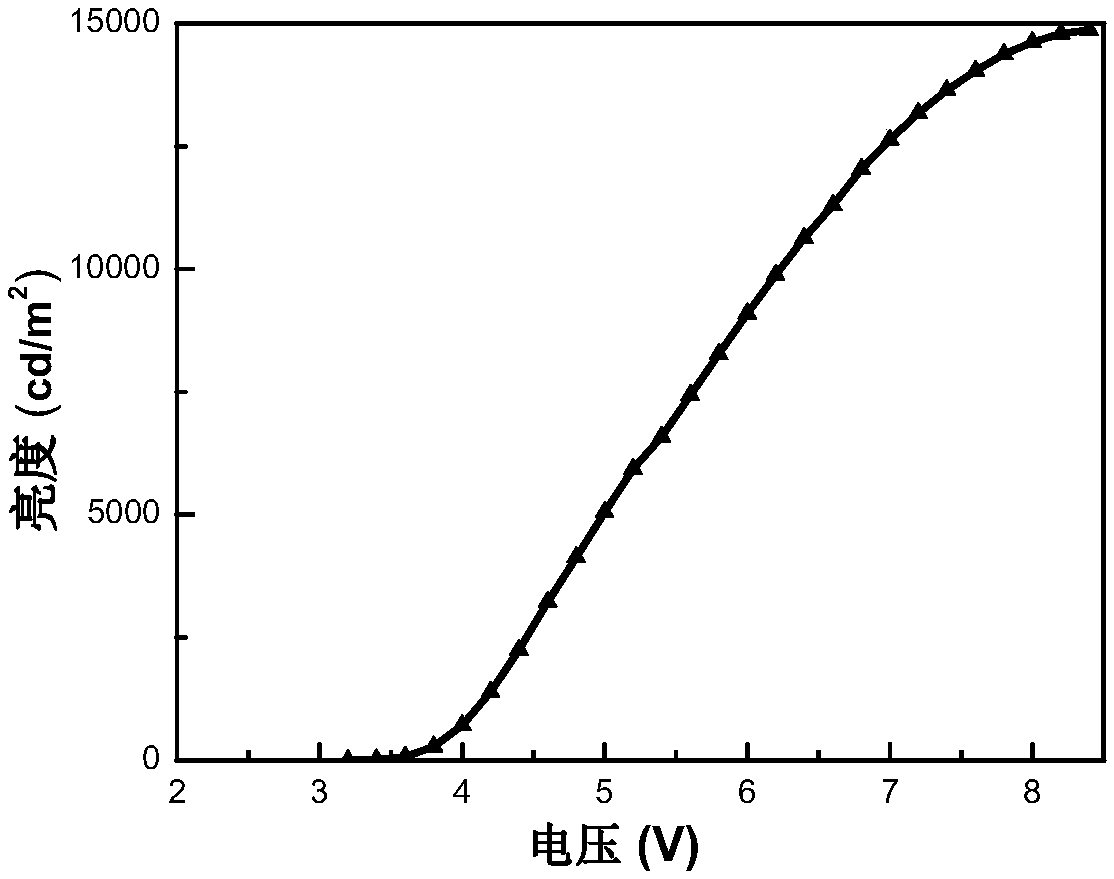
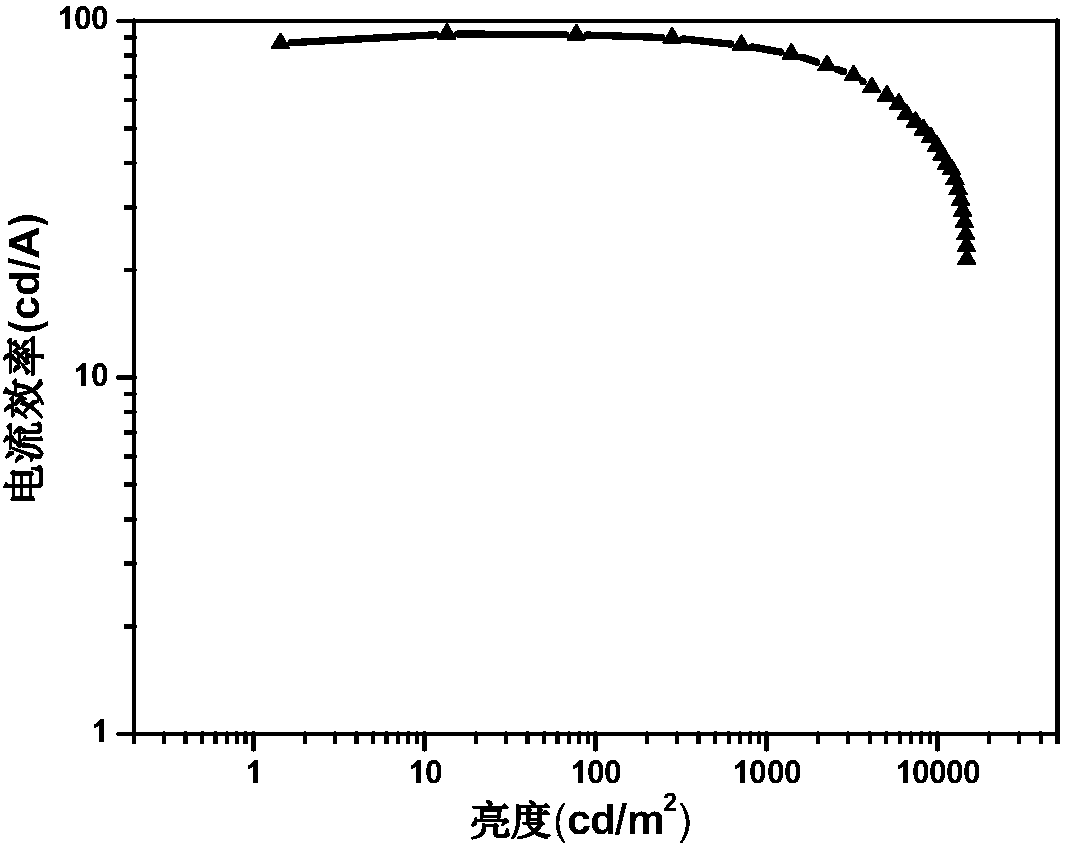
[0048] Embodiment 3 Preparation of iridium complex Stpip 1 organic electroluminescent device
[0049] Taking Stpip 1 as the light-emitting center of the light-emitting layer to prepare an organic electroluminescent device as an example, the preparation of the organic electroluminescent device of the present invention will be described. The structure of OLEDs device includes: substrate, anode, hole injection material, hole transport layer, organic light-emitting layer, electron transport layer, electron injection material and cathode. The substrate is glass, the anode is indium tin oxide, and the hole injection layer is 2,3,6,7,10,11-hexacyano-1,4,5,8,9,12-hexaazabenzene Phenanthrene HAT-CN (5nm), the evaporation rate is 0.05nm / s; the hole layer is made of 4,4'-cyclohexylbis[N,N-bis(4-methylphenyl)aniline TAPC material (50nm) , the evaporation rate is 0.05nm / s; the electron transport layer adopts 1,3,5-tris[(3-pyridyl)-3-phenyl]benzene TmPyPb (50nm), the evaporation rate is 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com