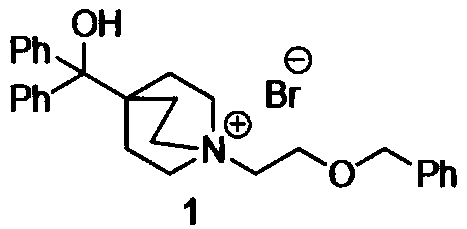
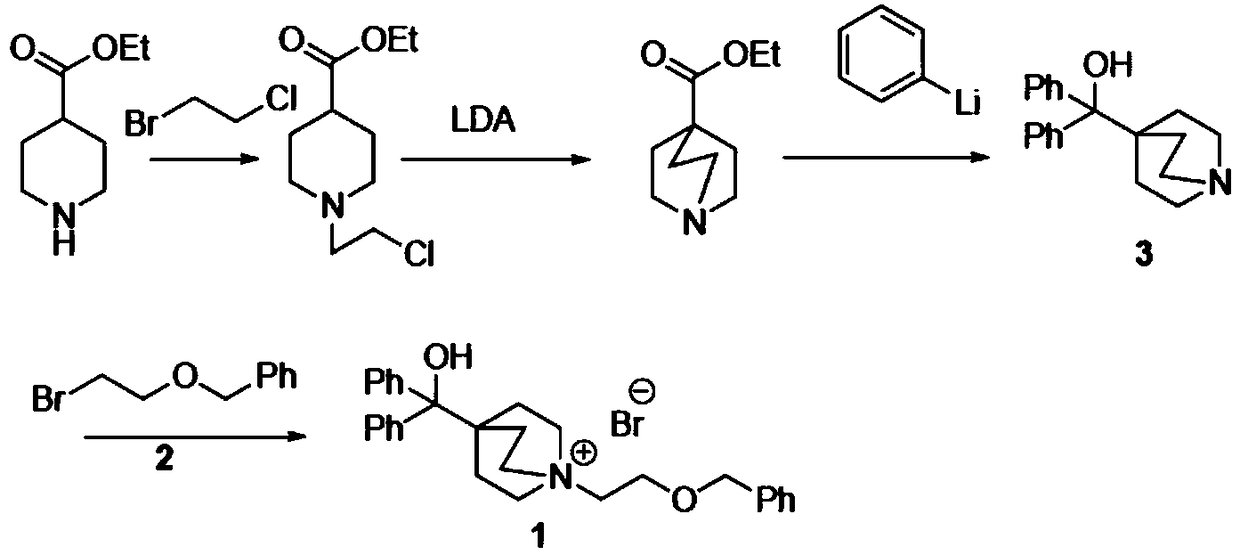
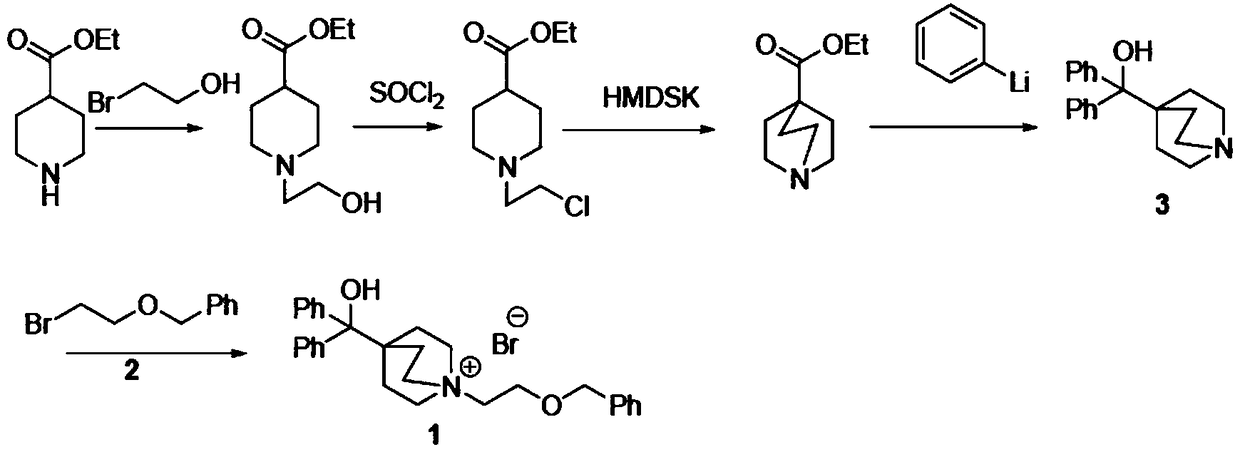
Novel method for synthesizing umeclidinium bromide
A technology of umeclidinium bromide and a synthetic method, which is applied in the field of pharmaceutical synthesis technology, can solve the problems of restricting the scale-up production of umeclidinium bromide, poor safety, and unresolved problems, so as to achieve improved reaction efficiency and yield, low cost, and high reaction efficiency. The effect of short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of 4-bromoquinuclidine Compound 5 was prepared from quinuclidin-4-ol (purchased from Hubei Wonder Chemical Co., Ltd.) by halogen substitution.
[0044] Add quinuclidin-4-ol (31.8 g, 0.25 mol) and phosphorus oxybromide (215 g, 0.75 mol) into the reaction flask, and raise the temperature to 80-90° C. for 4 h. Cool to 30-40°C, slowly pour the reaction solution into ice water, then add 10% aqueous sodium bicarbonate to adjust the pH value to 8-9, extract the product (200ml × 3) with dichloromethane, combine the organic phases, and use Washed with tap water and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness and then distilled under reduced pressure to obtain 38.3 g of oil (4-bromoquinuclidine), with a yield of 80.6%.
Embodiment 2
[0045] The preparation of embodiment 2 4-bromoquinucidine
[0046] Quinuclidin-4-ol (31.8 g, 0.25 mol) and 47% hydrobromic acid (500 ml) were added into the reaction flask, and the temperature was raised to reflux for 5 h. Cool to room temperature, add 10% aqueous sodium bicarbonate solution to adjust the pH value to 8-9, extract the product (200ml×3) with dichloromethane, combine the organic phases, wash with tap water, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated to dryness and then distilled under reduced pressure to obtain 40.1 g of oil (4-bromoquinuclidine), with a yield of 84.4%.
Embodiment 3
[0047] Example 3 Preparation of α,α-diphenyl-1-azabicyclo[2.2.2]octane-4-methanol
[0048] Add 4-bromoquinuclidine (38g, 0.200mol) and anhydrous tetrahydrofuran (400ml) into the reaction flask, start stirring, then add magnesium chips (10.7g, 0.440mol) and 1g iodine, and stir the reaction at room temperature under nitrogen protection 2h. Control the temperature below 10°C, add diphenyl ketone (40.1 g, 0.220 mol) in anhydrous tetrahydrofuran solution (100 ml) dropwise, after the drop is complete, naturally warm to room temperature and stir for 3 h, and monitor the completion of the reaction by TLC. Slowly pour the reaction solution into 250ml of saturated ammonium chloride solution to quench, separate the layers, extract the organic phase with 1N hydrochloric acid (200ml), combine the aqueous phases, add saturated potassium carbonate solution to adjust the pH to >9, and precipitate a solid. Filtration, the filter cake was washed with ethyl acetate (100ml), and after drying, 53...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com