Method for producing battery-grade ferric orthophosphate from titanium dioxide solid waste
A ferric orthophosphate, battery-level technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of small consumption, environmental pollution, high cost, reduce environmental pressure, reduce production costs, reaction more stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
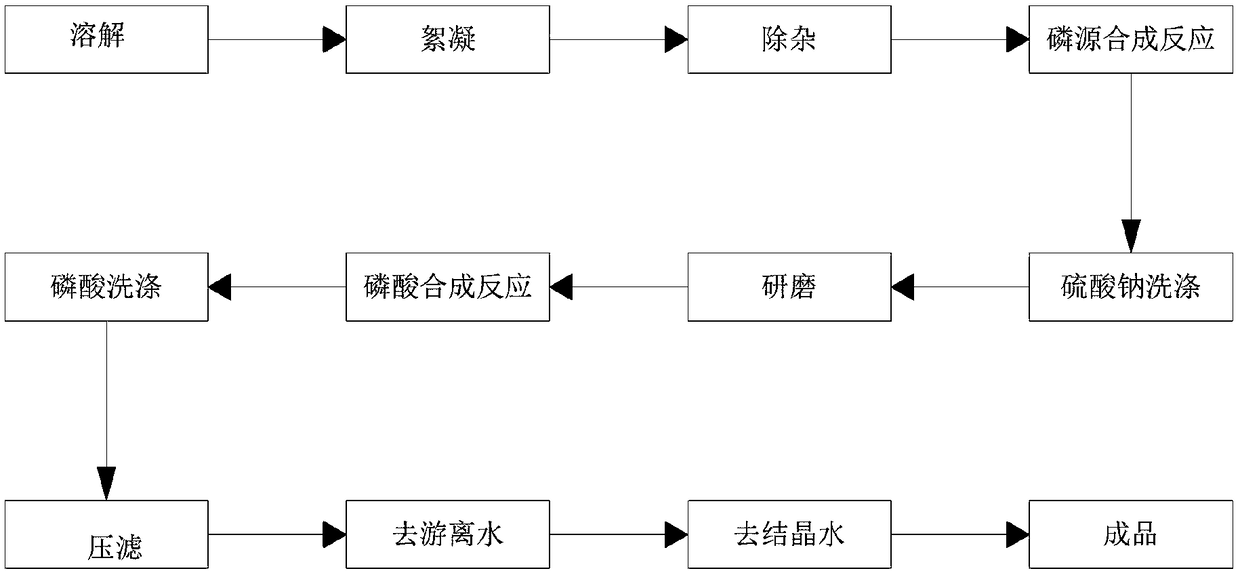
[0025] Example 1, such as figure 1 As shown, this embodiment provides a method for producing battery-grade ferric orthophosphate from titanium dioxide solid waste
[0026] Fully dissolve the titanium dioxide solid waste with water, and prepare a ferrous sulfate solution with a concentration of 25%. The main purpose of configuring the concentration between 25% is to better react with the phosphoric acid solution and achieve the purpose of full reaction. Then, add flocculant Finally, in this example, the flocculant is polyvinylamide and polyacrylamide, which are formed by copolymerization of ionic monomers and acrylamide in different proportions. Its molecular chain has a certain number of positive, negative, neutral charges and strong adsorption genes, so as to neutralize the charge on the surface of the colloidal particles of suspended solids in the treated water, destroy its stability, and destabilize it. The colloidal particles adsorb each other under the bridging effect of...
Embodiment 2
[0034] Embodiment 2, this embodiment provides a method for producing battery-grade ferric orthophosphate from titanium dioxide solid waste
[0035]Fully dissolve the titanium dioxide solid waste with water, and prepare a ferrous sulfate solution with a concentration of 27%. The main purpose of configuring the concentration between 27% is to better react with the phosphoric acid solution and achieve the purpose of full reaction. Then, add flocculant Finally, in this example, the flocculant is polyvinylamide and polyacrylamide, which are formed by copolymerization of ionic monomers and acrylamide in different proportions. Its molecular chain has a certain number of positive, negative, neutral charges and strong adsorption genes, so as to neutralize the charge on the surface of the colloidal particles of suspended solids in the treated water, destroy its stability, and destabilize it. The colloidal particles adsorb each other under the bridging effect of the polymer active gene, ...
Embodiment 3
[0043] Embodiment 3, this embodiment provides a method for producing battery-grade ferric orthophosphate from titanium dioxide solid waste
[0044] 30% test detection:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com