Zigzag type medium and wide bandgap small molecule electron donor materials based on thienoisobenzopyran and their applications
A technology of benzopyran and small molecules, which is applied in zigzag-type medium and wide bandgap small molecule electron donor materials based on thienoisobenzopyran and its application field, which can solve the problem that the types of donor materials are not wide enough
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The synthesis route of the D-A-D type aldehyde (9) conjugated derivative with thienoisobenzopyran as the electron-donating (A) unit and 5,6-bisfluorobenzothiadiazole as the electron-withdrawing (A) unit is as follows, detailed See Examples 1-6.
[0033]
[0034] Synthesis of 2-(tributyltin)-3-methoxythiophene
[0035]Compound 1 (40mmol, 4.56g) was placed in a 250mL dry three-necked flask, 70mL of dry tetrahydrofuran was added, and dissolved by magnetic stirring. Under the protection of nitrogen, n-butyllithium n-hexane solution (1.6M , 27mL), reacted for 1h, and naturally rose to room temperature for 0.5h. Then the temperature was lowered to -78°C, tributyltin chloride (44mmol, 14.32g) was added, and the reaction was raised to room temperature for 12h. Pour the reaction mixture into 90 mL of water, extract petroleum ether 2 to 3 times, 30 mL each time, collect the light yellow organic phase, and then wash the organic phase with saturated potassium carbonate solutio...
Embodiment 2
[0053] Thienoisobenzopyran-based A 1 -D-A-D-A 1 Characterization of properties of medium and wide bandgap small molecule electron donor materials and fabrication and performance testing of photovoltaic devices
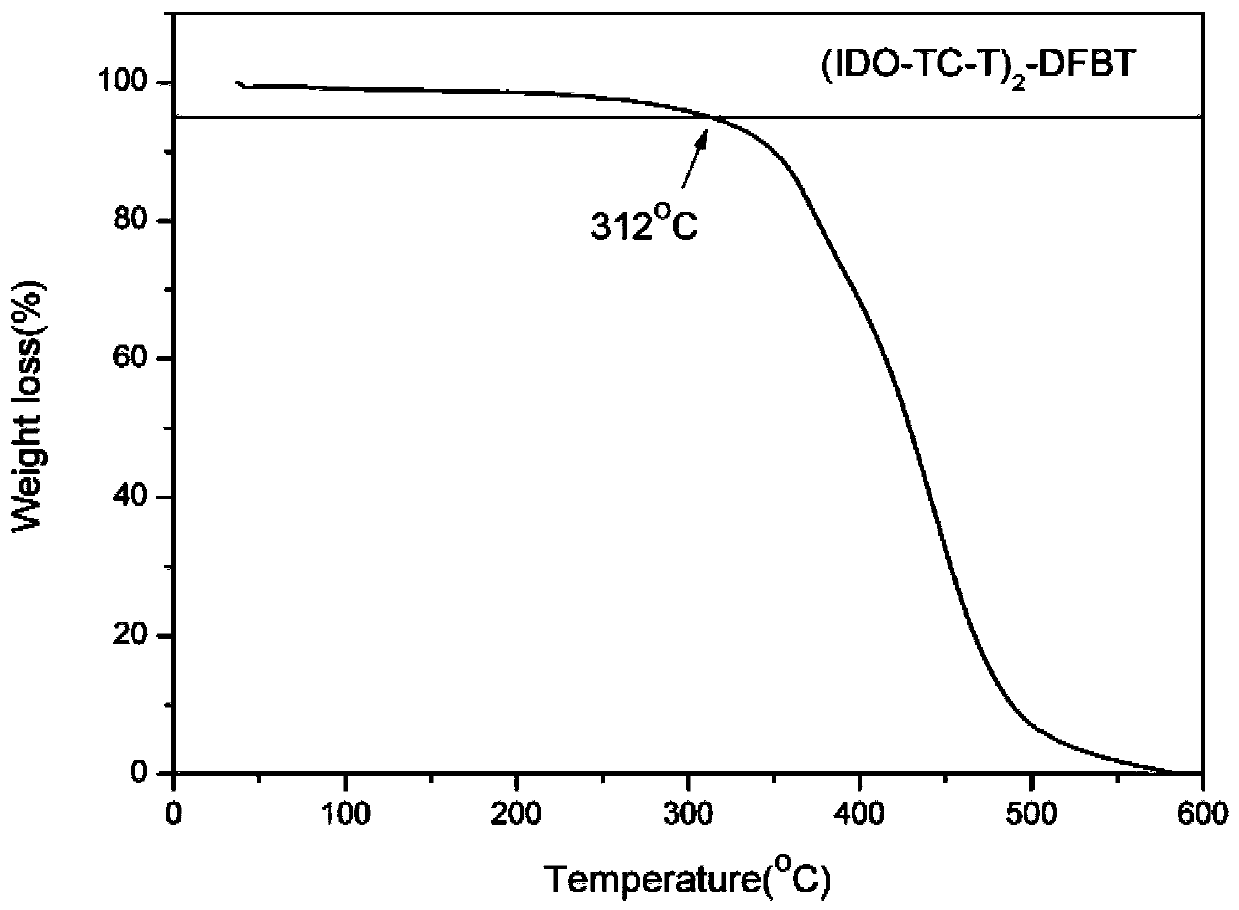
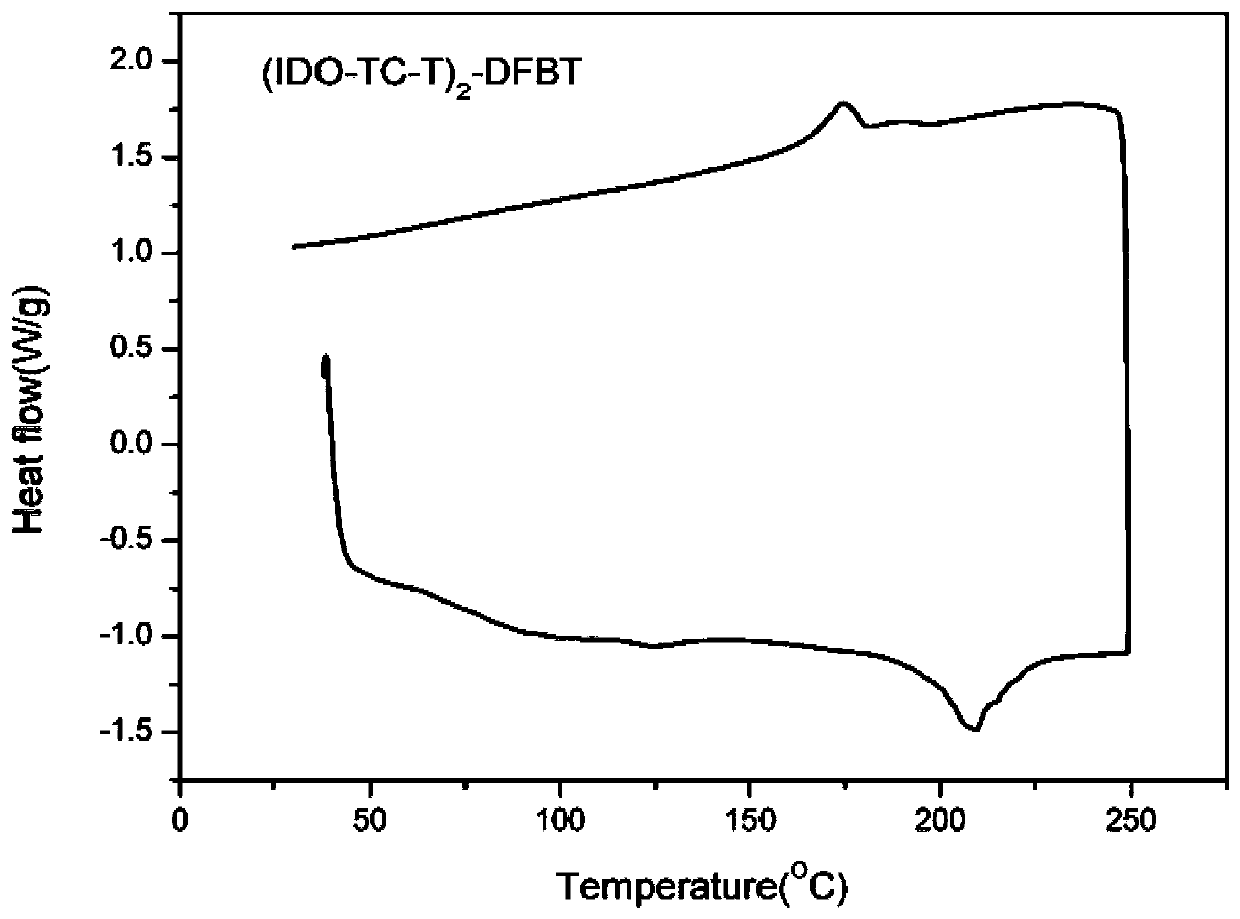
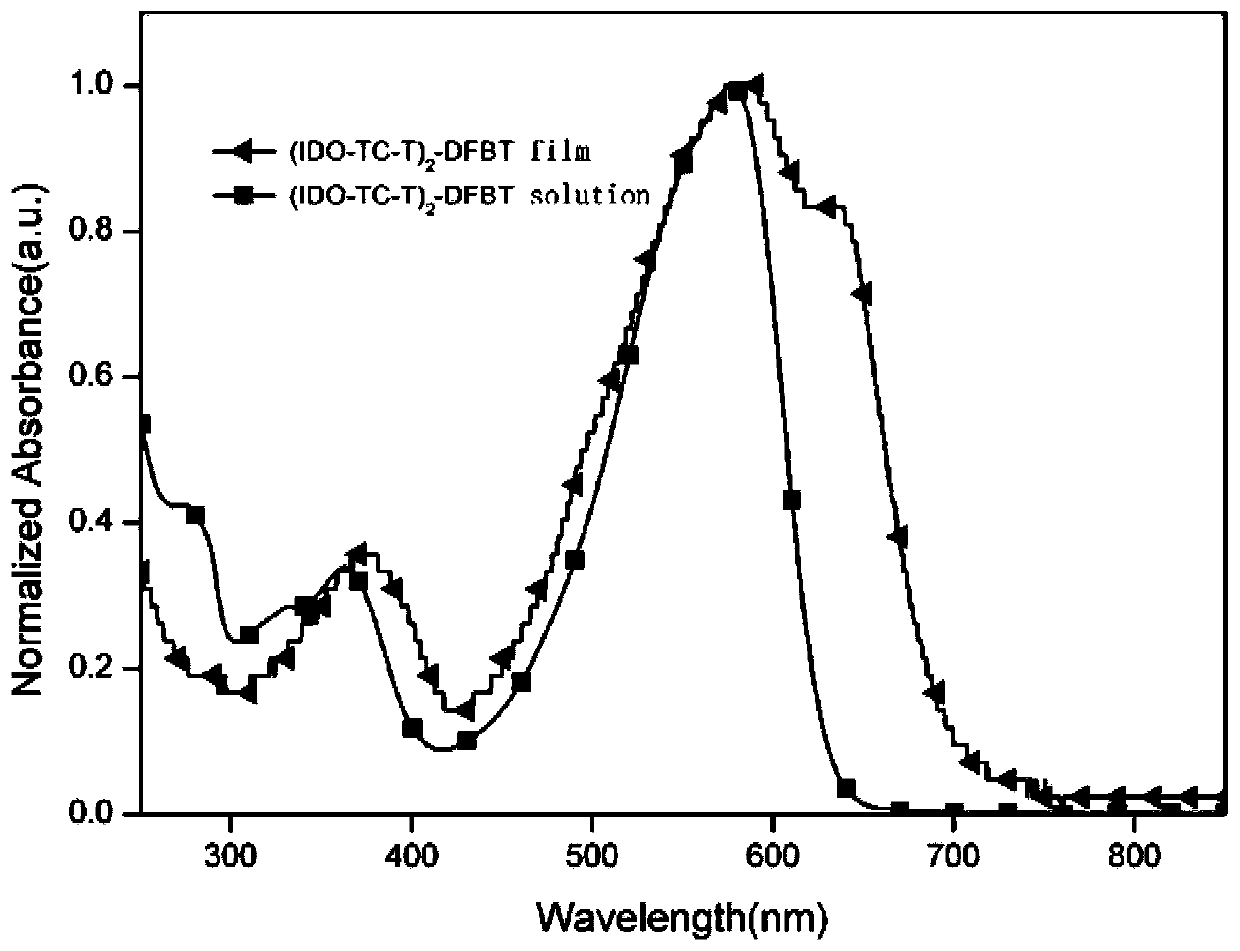
[0054] Novel Thienoisobenzopyran Derivative Donor Unit and Synthesis of All Intermediates in Its Synthesis 1 H NMR was measured by Bruker Dex-400 NMR instrument, and the ultraviolet-visible absorption spectrum of the zigzag type medium-wide bandgap small molecule electron donor material was measured by HP-8453 ultraviolet-visible spectrometer.
[0055] Based on thienoisobenzopyran, with A 1 -D-A-D-A 1 The organic solar cell device of the zigzag type medium-wide bandgap small molecule electron donor material of the frame includes: indium tin oxide (ITO) conductive glass anode layer, polystyrene sulfonate (PEDOT / PSS) anode modification layer, photoactive layer and cathode. The photoactive layer is composed of the small molecule photovoltaic material and PC 71 BM bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com