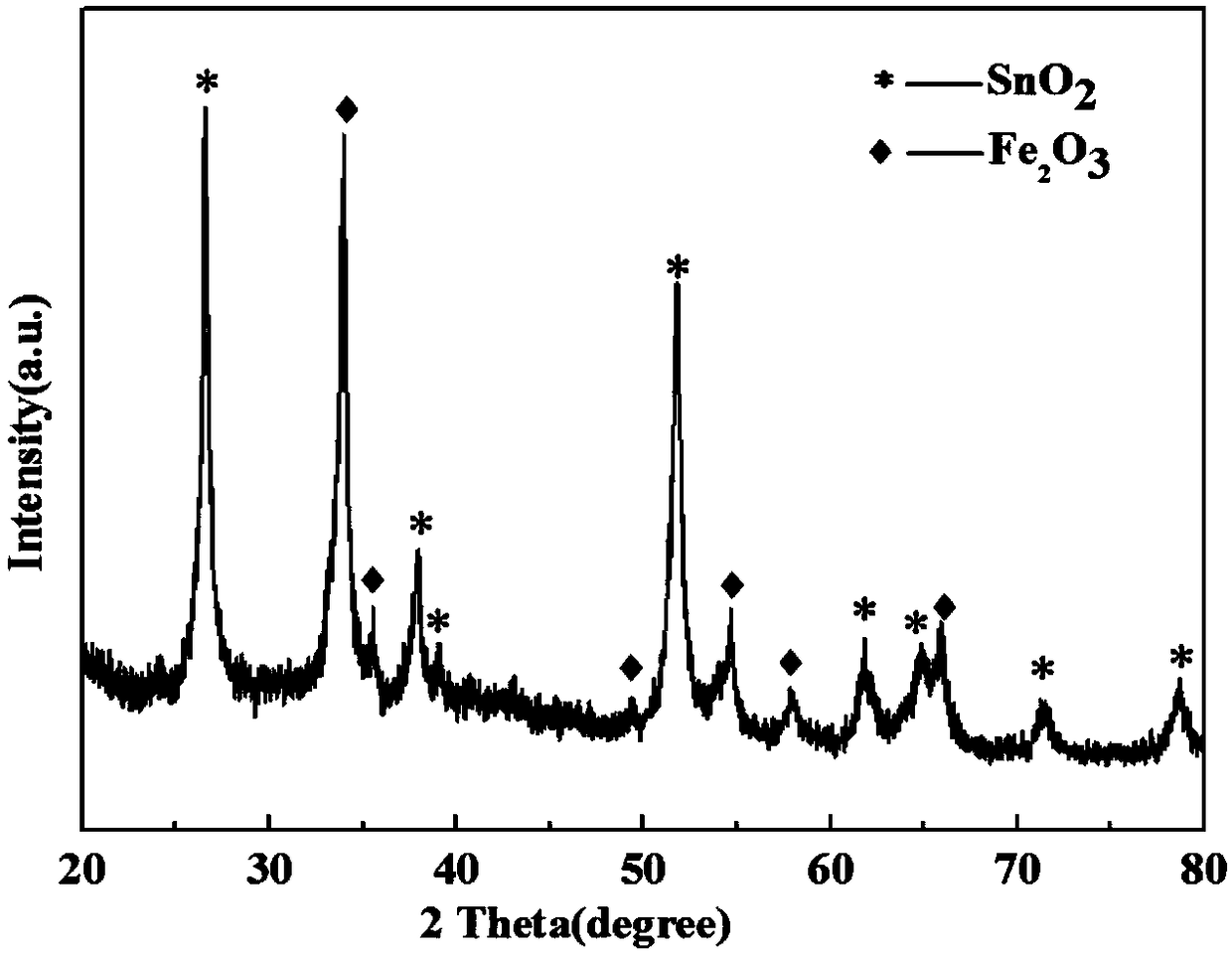
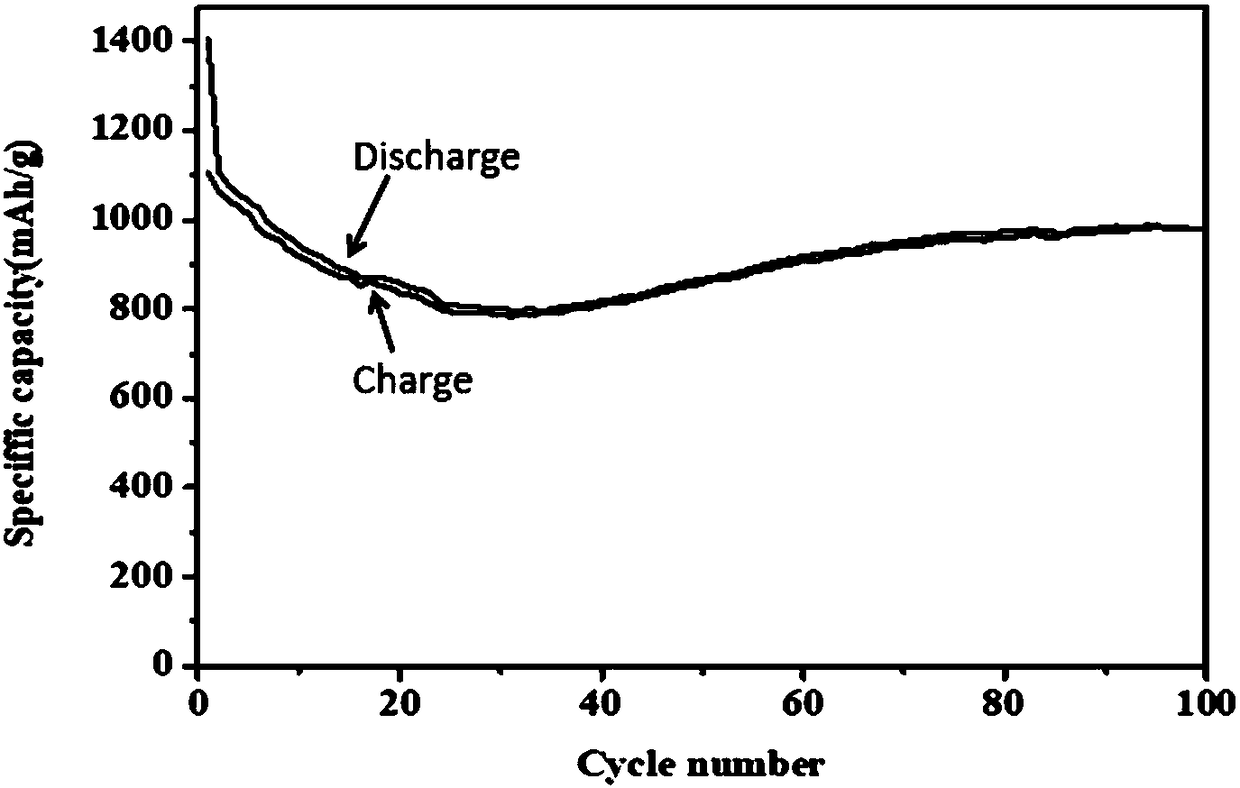
Carbon-doped nitrogen-coated tin oxide/iron oxide composite material, preparation method thereof, and lithium battery material
A composite material and nitrogen doping technology, which is applied in the field of lithium battery material preparation, can solve the problems of reducing energy density, difficult production conditions, and unstable volume formation of SEI film, etc., and achieves high chemical reaction activity and good reversibility of charge-discharge reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] According to the first aspect of the present invention, a method for preparing a carbon-doped nitrogen-coated tin oxide / iron oxide composite material is provided, comprising: dissolving binary lithium salt in anhydrous alcohol solution; adding iron salt and tin salt In the above solution; after 10-60 minutes, add the nitrogen source and carbon source into the solution; dry the above solution to obtain a solid; roast the solid to obtain a powder; wash the powder with water and centrifuge; dry the centrifuged solid to obtain a red color Solid powder; the red powder is roasted in a protective gas to obtain a carbon-doped nitrogen-coated tin oxide / iron oxide composite material.
[0032] Preferably, the binary lithium salt is two kinds of low melting point lithium salts;
[0033] Preferably, the binary lithium salt includes two of lithium bromide, lithium chloride, lithium nitrate and lithium acetate; more preferably, the binary lithium in the embodiment of the present inven...
Embodiment 1
[0049] In this method, lithium chloride and lithium nitrate are dissolved in absolute ethanol with a volume of 40 parts by mass of lithium nitrate at a ratio of 3:1, and anhydrous ferric chloride and tin tetrachloride are added to the above solution at a mass ratio of 40:1. , after stirring for 30 minutes, add urea and citric acid into the above solution according to the mass ratio of 1:1 and continue to stir thoroughly, then put the above solution in an oven to dry at 70°C for 24h, and then calcinate in air at 250°C for 4h, take out The product was washed with water and centrifuged three times, and the centrifuged solid was dried at 50°C and cooled to room temperature to obtain the primary product powder. Iron oxide composite electrode material.
Embodiment 2
[0051] In this method, lithium chloride and lithium nitrate are dissolved in absolute ethanol with a volume of 45 parts by mass of lithium nitrate at a ratio of 2:1, and anhydrous ferric chloride and tin tetrachloride are added to the above solution at a mass ratio of 40:2. , after stirring for 30 minutes, add urea and citric acid into the above solution according to the mass ratio of 2:1 and continue to stir fully, then put the above solution in an oven to dry at 75°C for 24h, then calcinate in air at 270°C for 3h, take out The product was washed with water and centrifuged three times, and the centrifuged solid was dried at 55°C and cooled to room temperature to obtain the primary product powder, and then the powder was put into nitrogen and roasted at 540°C for 3 hours to obtain carbon-doped nitrogen-coated tin oxide / Iron oxide composite electrode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com