A method for decomposing wolframite under oxygen pressure and preparing tungsten oxide and tungsten powder
A wolframite and tungsten oxide technology, applied in the field of chemical production, can solve the problems of high price, increase the cost of tungsten smelting, and pollute the environment, reduce production and operation costs, increase product added value, and reduce decomposition costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
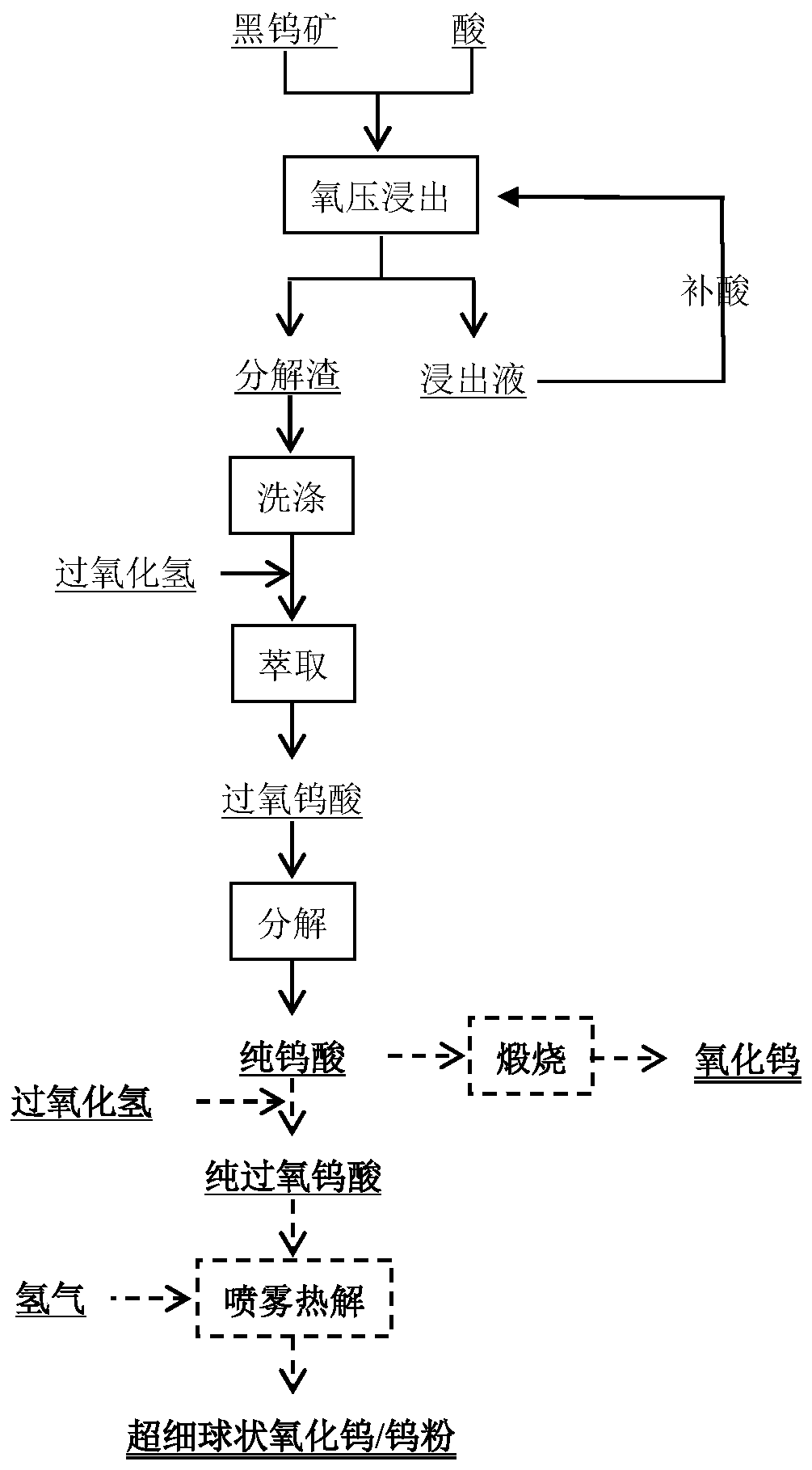
[0037] This example provides a method for decomposing wolframite by oxygen pressure and preparing tungsten oxide and tungsten powder. The raw material used in this method is wolframite with a grade of 40% and a particle size of 120 μm. The method is specifically as follows:
[0038] (1) Oxygen pressure leaching reaction: Add wolframite to a sulfuric acid solution with a concentration of 200g / L, the liquid-solid ratio of the system is 6:1, feed oxygen to control the oxygen pressure to 5Mpa, and carry out the leaching reaction at 150°C 3 hours, 99.4% of tungsten is converted into tungstic acid;
[0039] (2) Filtration and washing: filter the reaction product obtained in step (1) to obtain decomposition residue and leaching solution; add sulfuric acid to the leaching solution and return to step (1) as a raw material for leaching reaction;
[0040] (3) hydrogen peroxide extraction-decomposition and extraction of tungsten: wash the decomposed slag obtained in step (2) and mix it wi...
Embodiment 2
[0044] This example provides a method for decomposing wolframite by oxygen pressure and preparing tungsten oxide and tungsten powder. The raw material used in this method is wolframite with a grade of 10% and a particle size of 160 μm. The method is specifically as follows:
[0045] (1) Oxygen pressure leaching reaction: Add wolframite to a sulfuric acid solution with a concentration of 120g / L. The liquid-solid ratio of the system is 10:1. The leaching reaction is carried out under the condition of 180°C and controlled oxygen pressure of 10Mpa for 1 hour, and the 99.5 % of tungsten converted into tungstic acid;
[0046] (2) Filtration and washing: filter the reaction product obtained in step (1) to obtain decomposition residue and leaching solution; add sulfuric acid to the leaching solution and return to step (1) as a raw material for leaching reaction;
[0047] (3) hydrogen peroxide extraction-decomposition and extraction of tungsten: wash the decomposed slag obtained in ste...
Embodiment 3
[0052] This example provides a method for decomposing wolframite by oxygen pressure and preparing tungsten oxide and tungsten powder. The raw material used in this method is wolframite with a grade of 70% and a particle size of 60 μm. The method is specifically as follows:
[0053] (1) Oxygen pressure leaching reaction: adding wolframite to a sulfuric acid solution with a concentration of 160g / L, then adding solid tungstic acid accounting for 10% of the wolframite mass, the liquid-solid ratio of the system is 4:1, and the Oxygen is introduced into the leaching reaction for 5 hours at 150°C and the oxygen pressure is controlled at 6Mpa, and 99.3% of the tungsten is converted into tungstic acid;
[0054] (2) Filtration and washing: filter the reaction product obtained in step (1) to obtain decomposition residue and leaching solution; add sulfuric acid to the leaching solution and return to step (1) as a raw material for leaching reaction;
[0055] (3) hydrogen peroxide extractio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com