Clinical nutrient formulation special for sarcopenia and preparation method thereof
A nutritional formula and muscle attenuation technology, applied in food ingredients containing inorganic compounds, application, food ingredients containing acid, etc., can solve problems such as gastrointestinal function impact, nausea, and vomiting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
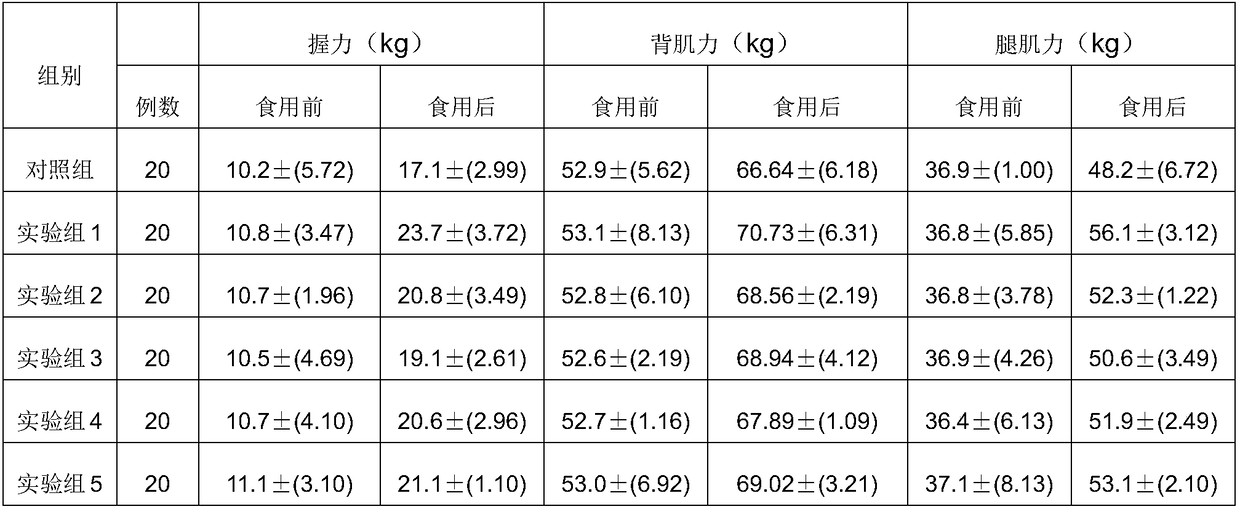
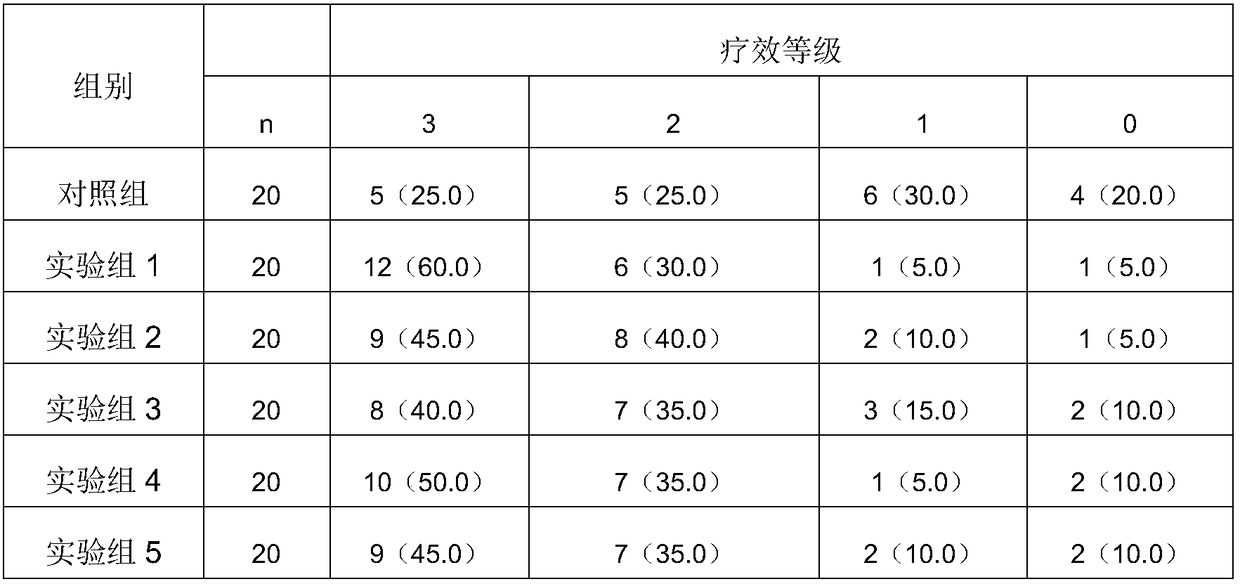
[0087] The specific clinical nutritional formula for sarcopenia of the present invention comprises 18.1 g of hydrolyzed whey protein powder, 5.45 g of branched-chain amino acids, 4.54 g of whole milk powder, 0.18 g of whole egg powder, 0.18 g of bovine colostrum freeze-dried powder, Taurine 0.12g; marine fish oligopeptide powder 0.09g, perilla oil 0.55g, coconut oil 2.36g, soybean oil 3.82g, olive oil 2.36g, medium chain triglycerides 0.29g; maltodextrin 32.16g, Resistant starch 12.73g; water-soluble dietary fiber 6.18g, insoluble dietary fiber 0.9g; calcium 0.2g, phosphorus 0.21g, potassium 0.08g, sodium 0.02g, magnesium 0.15g; iron 0.011g, iodine 0.00014g, zinc 0.007g ;Vitamin A0.0006g, β-carotene 0.000057g, vitamin D 3 0.000003g, Vitamin E 0.009g; Vitamin B 1 0.001g, Vitamin B 2 0.001g, Vitamin B 6 0.001g, Vitamin B 12 0.000002g, vitamin C 0.15g, pantothenic acid 0.003g, folic acid 0.000165g, niacin 0.0165g; dietary essence 1g; lily 0.05g, mint 0.05g, licorice 0.05...
Embodiment 2
[0090]The specific clinical nutritional formula for sarcopenia of the present invention comprises 18.1 g of hydrolyzed whey protein powder, 5.45 g of branched-chain amino acids, 4.54 g of whole milk powder, 0.18 g of whole egg powder, 0.18 g of bovine colostrum freeze-dried powder, Taurine 0.12g; marine fish oligopeptide powder 0.09g, perilla oil 0.55g, coconut oil 2.36g, soybean oil 3.82g, olive oil 2.36g, medium chain triglycerides 0.29g; maltodextrin 32.16g, Resistant starch 12.73g; water-soluble dietary fiber 6.18g, insoluble dietary fiber 0.9g; calcium 0.2g, phosphorus 0.21g, potassium 0.08g, sodium 0.02g, magnesium 0.15g; iron 0.011g, iodine 0.00014g, zinc 0.007g ;Vitamin A0.0006g, β-carotene 0.000057g, vitamin D 3 0.000003g, Vitamin E 0.009g; Vitamin B 1 0.001g, Vitamin B 2 0.001g, Vitamin B 6 0.001g, Vitamin B 12 0.000002g, vitamin C0.15g, pantothenic acid 0.003g, folic acid 0.000165g, niacin 0.0165g; dietary essence 1g; lily 0.05g, mint 0.05g, licorice 0.05g,...
Embodiment 3
[0093] The specific clinical nutritional formula for sarcopenia of the present invention comprises 18.1 g of hydrolyzed whey protein powder, 5.45 g of branched-chain amino acids, 4.54 g of whole milk powder, 0.18 g of whole egg powder, 0.18 g of bovine colostrum freeze-dried powder, Taurine 0.01g, marine fish oligopeptide powder 0.09g; perilla oil 0.55g, coconut oil 2.36g, soybean oil 3.82g, olive oil 2.36g, medium chain triglyceride 0.29g; maltodextrin 32.16g, Resistant starch 12.73g; water-soluble dietary fiber 6.18g, insoluble dietary fiber 0.9g; calcium 0.2g, phosphorus 0.21g, potassium 0.08g, sodium 0.02g, magnesium 0.15g; iron 0.011g, iodine 0.00014g, zinc 0.007g ;Vitamin A0.0006g, β-carotene 0.000057g, vitamin D 3 0.000003g, Vitamin E 0.009g; Vitamin B 1 0.001g, Vitamin B 2 0.001g, Vitamin B 6 0.001g, Vitamin B 12 0.000002g, vitamin C 0.15g, pantothenic acid 0.003g, folic acid 0.000165g, niacin 0.0165g; dietary essence 1g; lily 0.05g, mint 0.05g, licorice 0.05g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com