Photoelectric functional material based on phosphine sulfur/oxygen modifying phenazine
A technology for optoelectronic functional materials and synthesis methods, which can be applied in the fields of luminescent materials, compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, etc., and can solve problems such as the properties of the compounds themselves, the limitations of synthesis methods, and the instability of phenazine. , to achieve the effects of excellent optoelectronic properties, low toxicity of raw materials, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The present invention also discloses a method for synthesizing a photoelectric functional material based on phosphine sulfur / oxygen modified phenazine, the method comprising the following steps:
[0033] S1: Under the protection of nitrogen, dissolve phenazine in ethanol, dissolve sodium dithionite in distilled water, then inject the aqueous solution of sodium dithionite into the ethanol solution system of phenazine, and react under the conditions of heat reflux and shading After 1 to 5 hours, after cooling to room temperature, the flocs in the system were distilled under reduced pressure to obtain a white powder product, which was dried at room temperature to obtain the 5,10-dihydrophenazine intermediate. In S1, the molar ratio of phenazine to sodium dithionite is 1:8-1:10, the reaction temperature is 90-100° C., and the reaction time is 1-5 hours.
[0034] S2: Under nitrogen protection, dissolve the 5,10-dihydrophenazine intermediate obtained in step S1 in anhydrous t...
Embodiment 1
[0040] Synthesis of Phenazine Compounds Based on Aryl Phosphine Sulfur Modification
[0041]
[0042] Step 1, add 2.7g of phenazine to the reaction bottle, take another reaction bottle and add 26.1g of sodium dithionite, vacuumize and blow nitrogen for three times, inject 73mL of ethanol into the phenazine system under the protection of nitrogen, and inject 100mL of distillation into In the sodium dithionite system, after fully dissolving, inject the aqueous solution of sodium dithionite into the phenazine alcohol solution, perform light-shielding treatment, raise the temperature to 95° C. for reaction under nitrogen protection, and condense and reflux for 3 hours. After the reaction is completed, cool to room temperature, filter out the flocs, wash with distilled water, and dry to obtain the 5,10-dihydrophenazine intermediate without further purification.
[0043] Step 2, add 1.5g of 5,10-dihydrophenazine intermediate to the reaction bottle, vacuumize, blow nitrogen repeated...
Embodiment 2
[0051] Synthesis of Phenazine Compounds Modified by Arylphosphine Oxygen
[0052]
[0053] Step 5: Dissolve 0.5 g of the phosphine-containing aromatic derivative intermediate obtained in Step 3 in dichloromethane solution, then control the reaction system at 0°C, add 0.91 mL of 30% hydrogen peroxide, and keep the temperature at low temperature for ten minutes , return to room temperature, stir overnight at room temperature, and purify to obtain the target product (III).
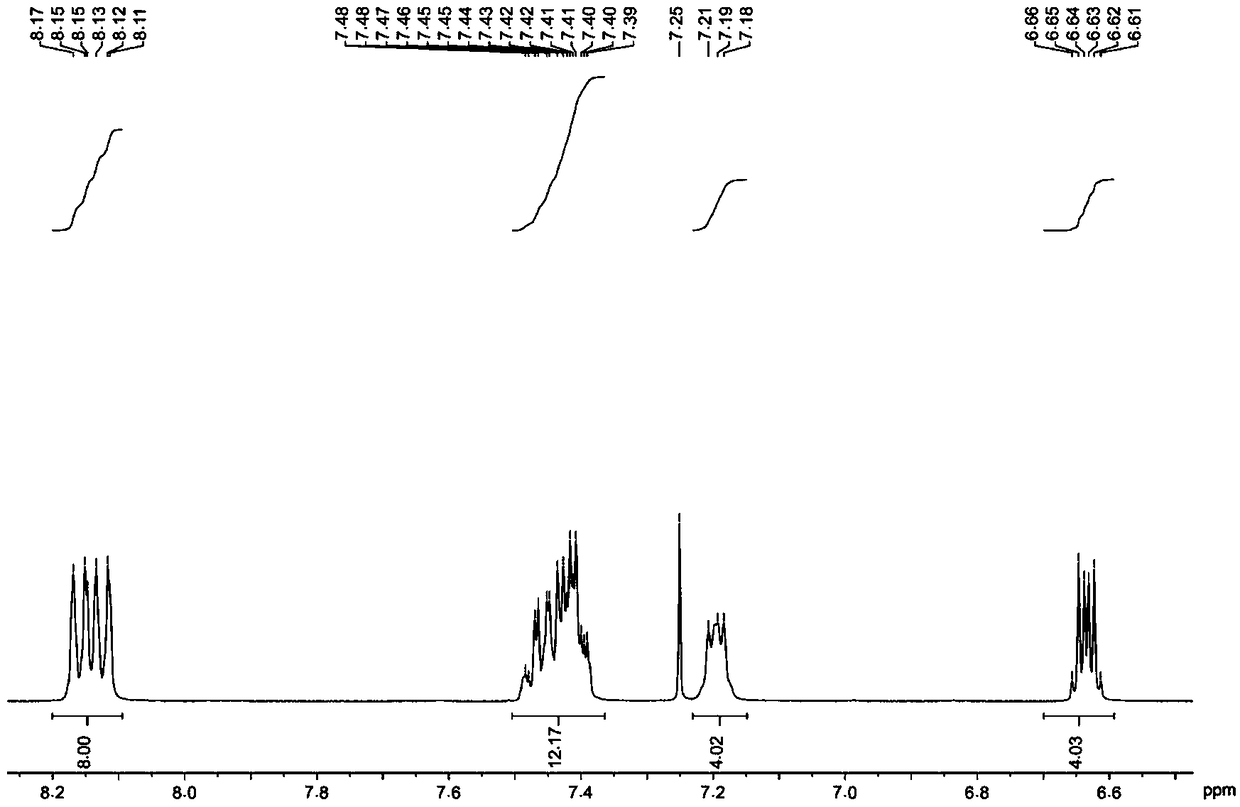
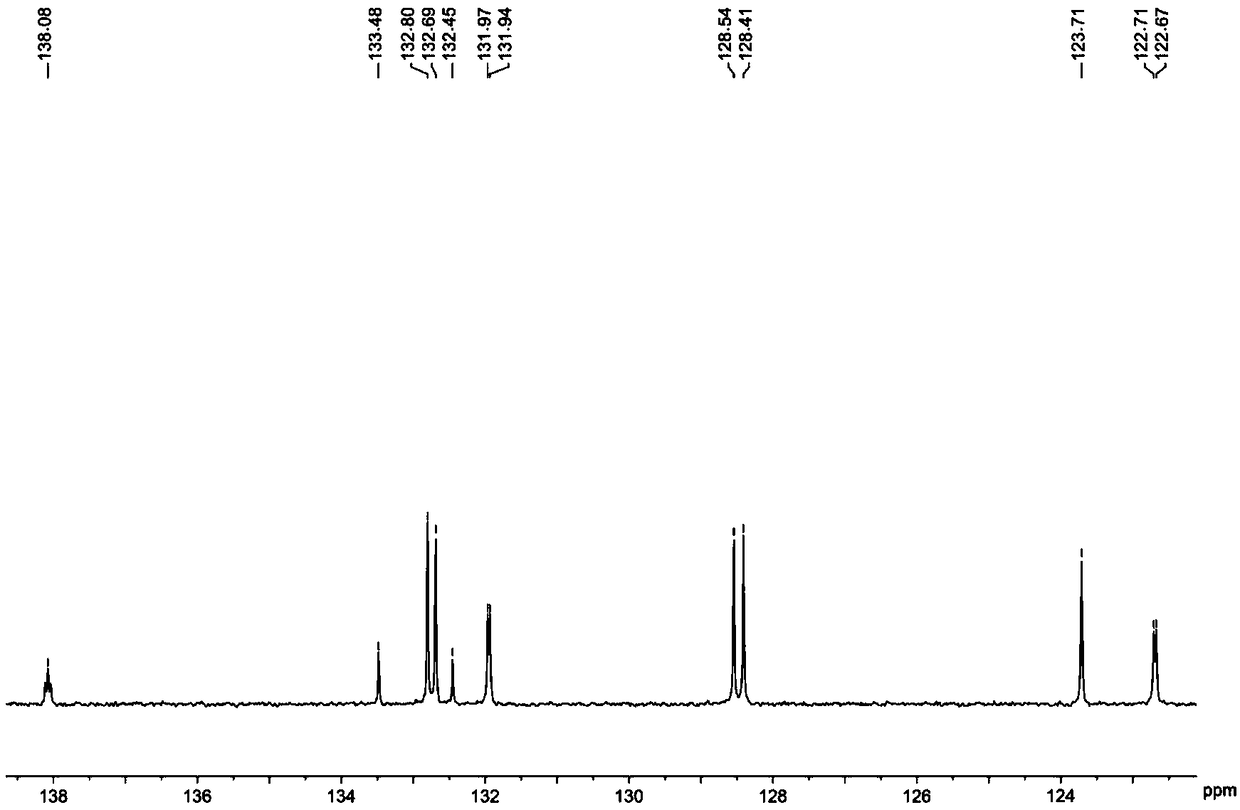
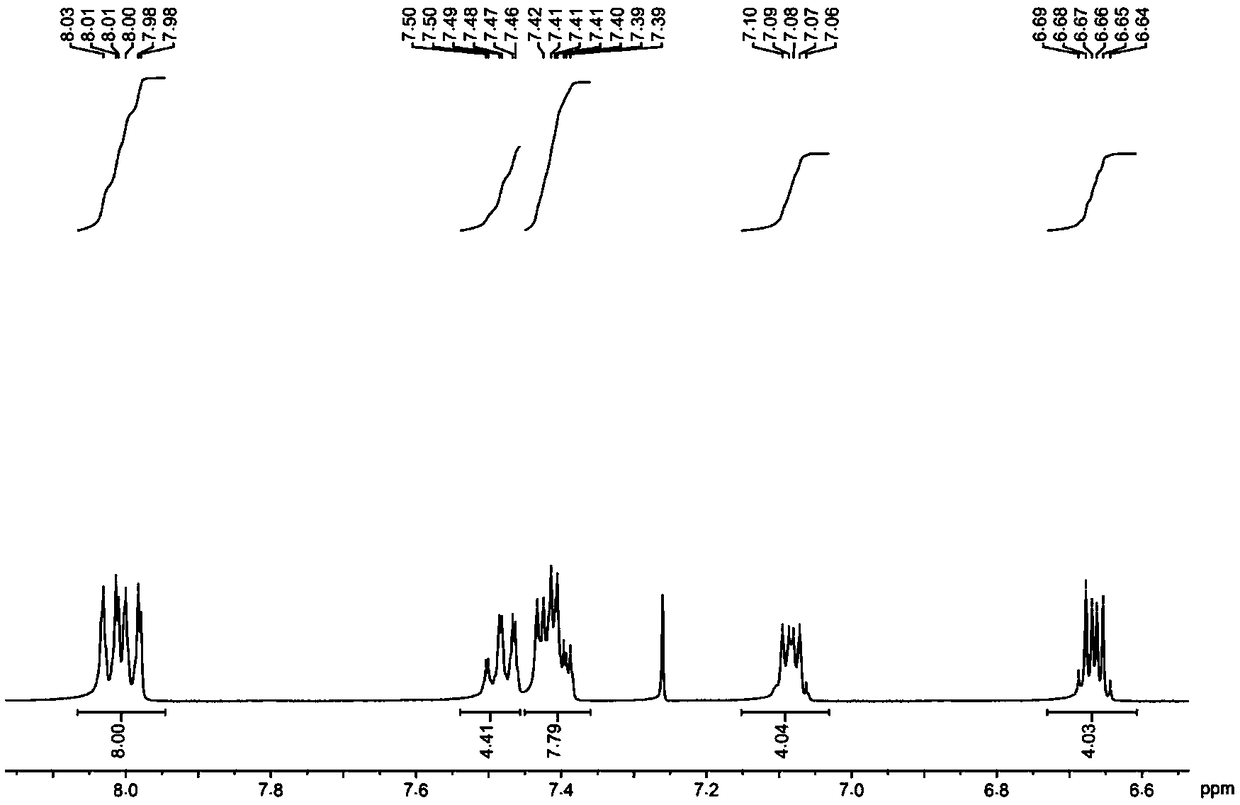
[0054] The hydrogen spectrogram and the carbon spectrogram of the obtained product are respectively as follows image 3 and Figure 4 As shown, the structural characterization data are as follows:
[0055] 1 H NMR (400MHz, CDCl 3 , ppm) δ8.03-7.98(m, 8H), 7.50-7.46(m, 4H), 7.42-7.93(m, 8H), 7.08(m, 4H), 6.69-6.64(m, 4H).
[0056] 13 C NMR (100MHz, CDCl3, ppm) δ137.24, 132.86, 132.77, 132.17, 132.14, 132.10, 130.81, 128.65, 128.52, 124.10, 122.80, 122.77.
[0057] MALDI-TOF m / z: 582.319.
[0058] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com