Method for producing beta-alanine and D-aspartic acid through conversion with biological enzyme method
A technology of aspartic acid and biological enzyme method, applied in the field of enzyme catalysis, can solve the problems of unsuitability for industrialization and low yield, and achieve the effects of reducing fermentation cost, convenient operation and saving labor cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
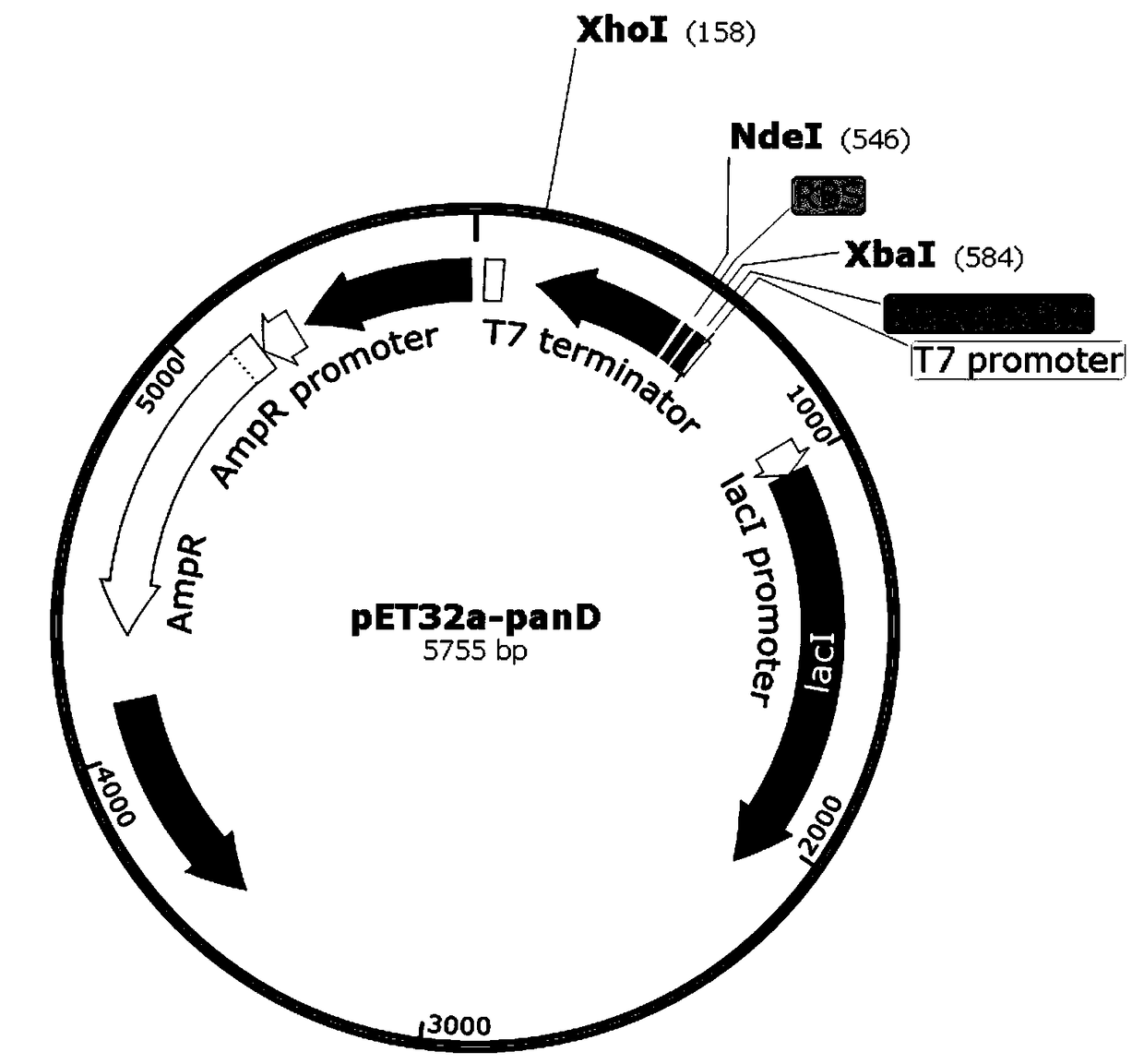
[0054] Embodiment 1: Construction of recombinant expression vector
[0055] The gene sequence of Bacillus subtilis L-aspartate-α-decarboxylase (PanD) obtained from the NCBI database, in order to delete the fusion expression located between the multiple cloning site and the T7 promoter on the expression vector Tag the protein to avoid the impact on the recombinant PanD protease activity, so add restriction enzyme sites NdeI (CATATG) and XhoI (CTCGAG) at both ends of the PanD gene sequence, where the 5th end of the PanD gene is NdeI, and the 3rd end is XhoI . as follows:
[0056] GC CATATG TACCGTACCATGATGTCTGGTAAACTGCACCGTGCTACCGTTACCGAAGCTAACCTGAACTACGTTGGTTCTATCACCATCGACGAAGACCTGATCGACGCTGTTGGTATGCTGCCGAACGAAAAAGTTCAGATCGTTAACAACAACAACGGTGCTCGTCTGGAAACCTACATCATCCCGGGTAAACGTGGTTCTGGTGTTATCTGCCTGAACGGTGCTGCTGCTCGTCTGGTTCAGGAAGGTGACAAAGTTATCATCATCTCTTACAAAATGATGTCTGACCAGGAAGCTGCTTCTCACGAACCGAAAGTTGCTGTTCTGAACGACCAGAACAAAATCGAACAGATGCTGGGTAACGAACCGGCTCGTACCATCCTGTAA CTCGAG CG...
Embodiment 2
[0060] Embodiment 2: the acquisition of engineering bacteria
[0061] The operation steps are as follows:
[0062] (1) Take a tube of 50 μL E.coli Rosetta (DE3) competent cells and place them on ice. After the competent cells are completely thawed, gently blow with a pipette to suspend the competent cells evenly.
[0063] (2) Pipette 1 μL of the pET32a-panD recombinant plasmid, add it to E.coli Rosetta (DE3) competent cells, mix gently by pipetting, and let stand on ice for 30 minutes.
[0064] (3) Heat shock in a metal bath at 42°C for 90 seconds, and immediately place it on ice for 2 minutes.
[0065] (4) Add 500 μL of LB medium without antibiotics, recover at 37° C., 220 rpm for 1 hour.
[0066] (5) Centrifuge at 4000rpm for 5min, pipette 300μ supernatant with a pipette gun, discard, and gently pipette to suspend the cells.
[0067] (6) Under a sterile environment, use a coating rod to evenly spread the cells on the LB solid medium plate (Amp resistance), control the tem...
Embodiment 3
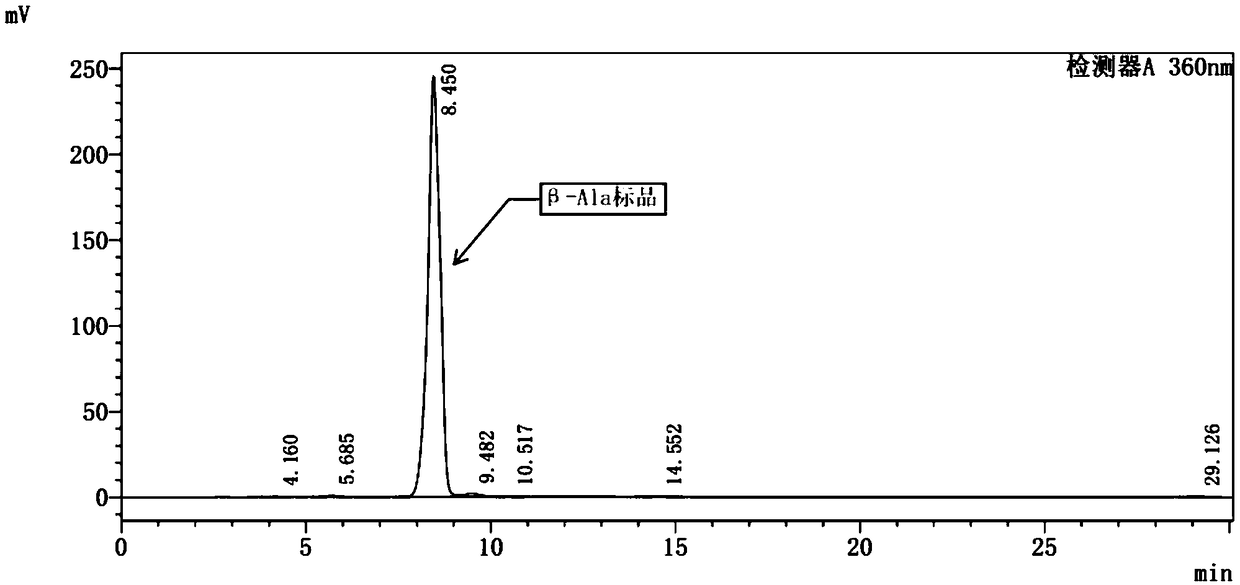
[0069] Embodiment 3: Fermentation and biotransformation of engineering bacteria DPD to synthesize β-alanine
[0070] (1) Under sterile conditions, inoculate 0.1% to 1% of the inoculum from the engineered bacteria DPD glycerol tube into the primary seed medium, cultivate at 37°C and 220 rpm until the OD600 reaches 3.0 to 4.0, and stop the cultivation.
[0071] (2) Transfer the primary seed bacterial liquid to the secondary seed culture medium according to the inoculum amount of 4%-10%, and cultivate for 3-5 hours at a temperature of 37° C. and a rotational speed of 220 rpm.
[0072] (3) Transfer the cultured secondary seed bacterial liquid into a 5L fermenter (containing 3L fermentation medium) by flame inoculation at an inoculum size of 1% to 8%, initial fermentation parameters: temperature 37°C, rotating speed 200rpm, pH=7.0, ventilation rate 50L / h, tank pressure 0.05MPa; as the fermentation time prolongs, the bacterial concentration gradually increases, and the dissolved oxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com