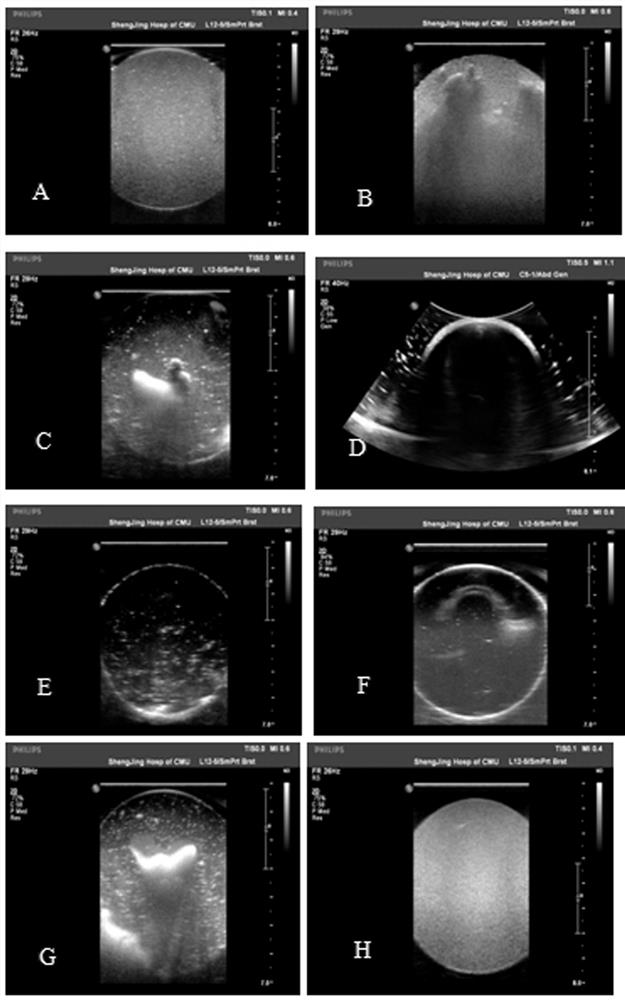
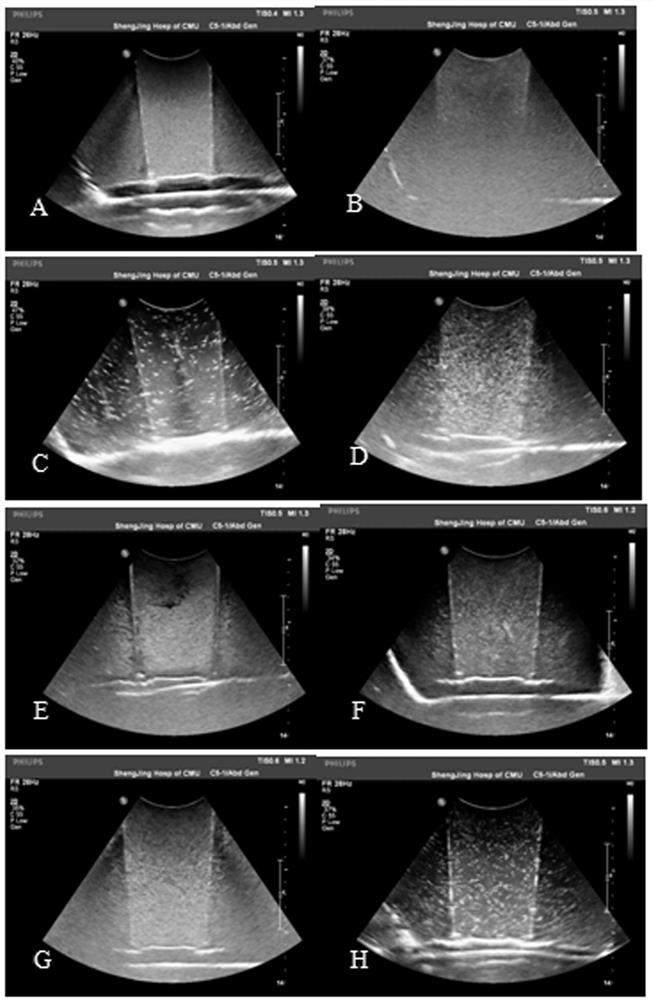
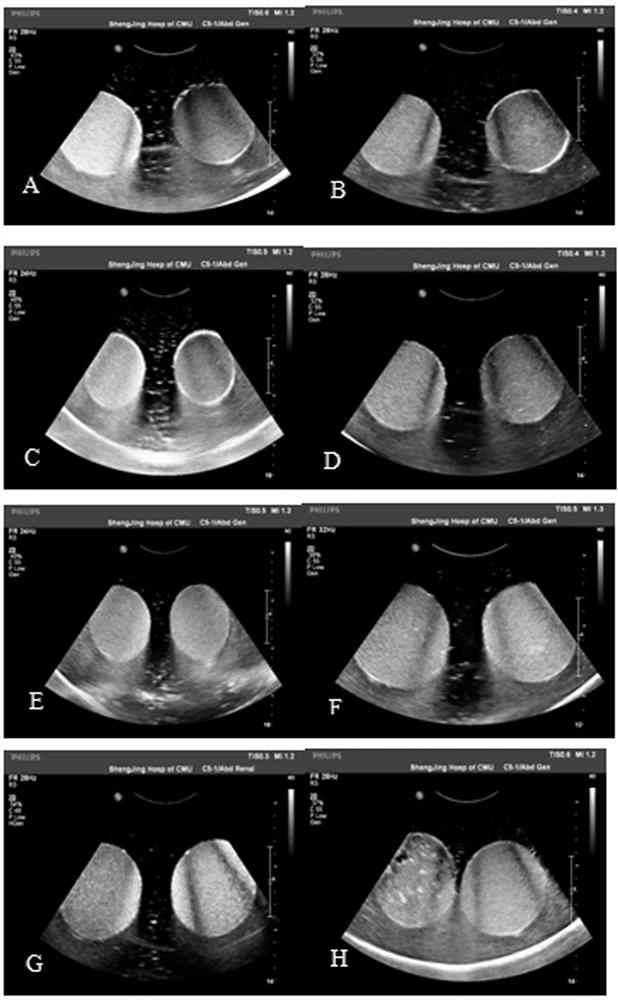
A ready-to-use liquid oral gastric ultrasound contrast agent formula and preparation method
A developer and ultrasonic technology, applied in the direction of echo/ultrasonic imaging agent, liquid delivery, emulsion delivery, etc., can solve the problems of complex preparation methods, difficulty in mixing uniformly, and elevated blood calcium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 1. Formula.
[0050] Microcrystalline Cellulose PH102(D 50 : 100 μm) mass fraction is 4%, colloidal microcrystalline cellulose Avicel CL-611 mass fraction is 3.0%, xanthan gum mass fraction is 0.3%, glycerin mass fraction is 10%, methyl paraben mass fraction is The massfraction is 0.1%, the sucralose massfraction is 0.01%, the orange flavor essence massfraction is 0.08%, the Simethicone emulsion (30%) massfraction is 0.1%, the deionized water massfraction (is divided into two parts: 85% and 15%) was 82.41%.
[0051] 2. Preparation method.
[0052] 1) Take about 85% deionized water, add methylparaben and glycerin, stir and disperse at 200rpm, and dissolve or disperse evenly.
[0053] 2) Add colloidal microcrystalline cellulose (Avicel CL-611) and xanthan gum in sequence, stir and disperse evenly at 200rpm, then use FLUKA-FA28 high-shear homogenizer at a homogenization speed of 13000rpm for high-shear homogenization , and homogenize until the material is fully hydrate...
Embodiment 2
[0059] 1. Formula.
[0060] Microcrystalline Cellulose PH102(D 50 :100 μm) mass fraction is 4.0%, colloidal microcrystalline cellulose Avicel RC-581 mass fraction is 3.5%, xanthan gum mass fraction is 0.2%, glycerin mass fraction is 7.5%, sodium benzoate mass fraction is 0.1%, three The mass fraction of sucralose is 0.01%, the mass fraction of mint flavor essence is 0.08%, the mass fraction of simethicone emulsion (30%) is 0.1%, and the mass fraction of deionized water (divided into two parts: 85% and 15%) is 84.51%.
[0061] 2. Preparation method.
[0062] 1) Take about 85% deionized water, add sodium benzoate and glycerin, stir and disperse at 200rpm to dissolve or disperse evenly.
[0063] 2) Add colloidal microcrystalline cellulose (Avicel RC-581) and xanthan gum in turn, stir and disperse evenly at 200rpm, then use FLUKA-FA28 high-shear homogenizer at a homogenization speed of 13000rpm for high-shear homogenization , and homogenize until the material is fully hydrated ...
Embodiment 3
[0069] 1. Formula.
[0070] Microcrystalline Cellulose PH102(D 50 : 100 μm) mass fraction is 4.0%, colloidal microcrystalline cellulose Avicel RC-501 mass fraction is 2.5%, xanthan gum mass fraction is 0.3%, glycerin mass fraction is 10.0%, sodium butylparaben mass fraction is 0.1 %, the mass fraction of aspartame is 0.1%, the mass fraction of banana flavor essence is 0.1%, the mass fraction of simethicone emulsion (30%) is 0.1%, the mass fraction of deionized water (divided into two parts: 85% and 15%) was 82.8%.
[0071] 2. Preparation method.
[0072] 1) Take about 85% deionized water, add sodium butylparaben and glycerin, stir and disperse at 200rpm to dissolve or disperse evenly.
[0073] 2) Add colloidal microcrystalline cellulose (Avicel RC-501) and xanthan gum in turn, stir and disperse evenly at 200rpm, then use FLUKA-FA28 high-shear homogenizer at a homogenization speed of 13000rpm for high-shear homogenization , and homogenize until the material is fully hydrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com